Fig. 5.1
Outline of the hydrodynamic mechanism by which (a) stimuli activate intradental nerves to cause pain and (b) subsequent resolution following natural desensitisation and treatment (Acknowledgement Orchardson and Gillam 2006)
1.
Dentin blocking agents that occlude patent (open) tubules (fluoride, strontium salts, oxalate, calcium phosphate, restorative materials, etc.) and as a consequence reduce any stimulus-evoked fluid movements within the dentin tubule
2.
Nerve desensitisation agents that reduce intradental nerve excitability (e.g. potassium ions, guanethidine) in order to prevent a response from intradental nerves to the stimulus-evoked fluid movements within the dentin tubules
It should be acknowledged that in vitro results demonstrating superiority of the various products under examination should not be extrapolated into making claims on the efficacy of these products without first undergoing extensive clinical evaluation. There are however a vast array of products currently out on the commercial market with various claims of clinical efficacy in reducing DH, although currently there does not appear to be a gold standard product or therapy universally accepted by clinicians to treat the condition (see Chap. 6).
Application of these successfully tested products may either involve ‘in-office’ procedures by a clinician using a restorative approach (for example, restorative materials in the form of dentin bonding agents, glass ionomer cements (GIC), and periodontal surgical techniques) or by a clinician recommending an over-the-counter (OTC) approach (involving toothpastes, gels, mouthwashes).
5.2 Dentin Blocking Agents
Currently the hydrodynamic theory (Brännström 1963) is generally considered to be the mechanism of choice although other alternative mechanisms of stimulus transmission cannot be ruled out, since some clinical phenomena cannot be explained solely by this theory (Gillam 1992; Orchardson and Gillam 2006) (Fig. 5.1).
There are a number of products commercially available for the treatment of DH these include both in-office applied and over-the-counter products. The proposed mode of action for most of these products has been established using in vitro, animal, in situ and in vivo studies (Orchardson and Gillam 2006 Tables 5.1a and 5.1b).
Table 5.1a
Characteristics of selected occluding toothpastes
|
Product
|
Composition
|
Proposed mode of action
|
|---|---|---|
|
SensiStat®
|
Contains arginine in combination with calcium and bicarbonate/carbonate
|
The arginine complex binds to the tooth surface and allows the calcium carbonate to slowly dissolve and release calcium. Limited in vitro and in vivo studies have been published in support of both laboratory and clinical claims for the product. Tubular occlusion
|
|
Colgate Pro-Argin™
|
Hydroxyapatite, sodium monofluorophosphate (MFP)
|
Recent in vitro and in vivo studies have been published in support of both laboratory and clinical claims for the product. Tubule occlusion
|
|
SensiShield®
|
Composed of calcium phosphorus, sodium and silica (calcium sodium phosphosilicate)
|
NovaMin® in contact with saliva and water reacts and releases Ca and PO4 ions. Sodium ions in the NovaMin particles exchange with hydrogen cations which in turn allows the calcium and phosphate ions to be released. A calcium phosphate layer is formed and subsequently crystallises into hydroxycarbonate apatite. The exposed dentin surface appears to act as a nucleation site for these ions to form hydroxycarbonate apatite and bypasses the intermediate phase of ACP formation. Mainly in vitro support for occlusion of dentine tubules, limited published clinical data supporting clinical efficacy of the product. Tubular occlusion
|
|
(NovaMin®)
|
||
|
Amorphous calcium phosphate (ACP)
|
ACP is inorganic in nature and is made by combining soluble salts of calcium and phosphate through a two-phase system containing Ca in one part and PO4 in another. When mixed together they react to form an amorphous phosphate material that precipitates on to the tooth surface
|
ACP is highly soluble and susceptible to acid attack, and as such the ACP is not protected and as it has no delivery system, it has lower substantivity. It has lower substantivity. ACP is not bioavailable after the product is rinsed away. Previously incorporated in Enamelon toothpaste (no longer available) which relied on a dual-chamber system in the toothpaste tube. The product is now available in Enamel Care toothpaste (Church and Dwight). Limited and equivocal published data for effectiveness of ACP in the treatment of dentin hypersensitivity
|
|
Recaldent (CPP–ACP)
|
Casein phosphates (CPP) are peptides derived from milk protein casein that are complexed with calcium (Ca) and phosphate (PO4). In this complex the CPP maintains the Ca and PO4 ions in an amorphous form (ACP). The milk-derived peptide containing amorphous Ca and PO4 is the driving mechanism that binds to plaque, bacteria and the tooth surface
|
CPP–ACP uses peptides derived from the milk protein casein to maintain Ca and PO4 in an amorphous calcium phosphate. The CPP binds to surfaces such as plaque, bacteria and soft tissue providing a bioavailable Ca and PO4 at the surface of the tooth without precipitation. The ACP is released during acidic challenges. Stabilisation of ACP by the CPP ensures the delivery of Ca and PO4 ions into the tooth structure before the ions crystallise. Most in vitro and in vivo studies support the product’s anticaries benefit, however there does not appear to be any published clinical support on its effect in reducing dentin hypersensitivity
|
|
Nanit®active (Henkel)
|
Hydroxyapatite, sodium monofluorophosphate (MFP)
|
According to Henkel’s product literature Nanit®active induces a process referred to as neomineralisation. The Nanit®active nanoparticles react with the calcium and phosphate ions in saliva, and a new protective layer is formed on the tooth surface (1–2 μm). Limited data available at present. Tubular occlusion
|
Table 5.1b
Characteristics of selected dentin blocking toothpastes
|
Product
|
Composition
|
Proposed mode of action
|
|---|---|---|
|
Blanx® Biorepair®
|
Hydroxyapatite, sodium monofluorophosphate (MFP)
|
Limited published data available at present. Tubular occlusion
|
|
Strontium salts (Sensodyne)
|
Strontium chloride (original), no fluoride
|
Hydrated technology. Published in vitro and in vivo studies supporting both the proposed mode of action and clinical effectiveness of both the acetate and chloride variants of the product. Tubular occlusion
|
|
Strontium actate, sodium monofluorophosphate (MFP)
|
||
|
Stannous fluoride
|
Stannous fluoride
|
Anhydrous technology. Uses hexametaphosphate to limit stains associated with the use of stannous ions. Two clinical studies in support of claims. Tubular occlusion
|
|
Crest® ProHealth™
|
||
|
Colgate SnF2
|
Stannous fluoride, potassium nitrate (5 %)
|
Dual-chamber delivery system. Published in vitro and in vivo studies supporting both the proposed mode of action and clinical effectiveness of the product. Presence of potassium would indicate its use as a nerve desensitiser; however in vitro studies tubule occlusion is in evidence. According to Mason et al. (2010) this product is no longer commercially available
|
|
Amine fluoride (elmex SENSITIVE)
|
Amine fluoride (olaflur)
|
Amine fluoride leads to the formation of a protective layer on the dentin containing calcium fluoride, which helps promote remineralisation and tubular occlusion. Limited published data available. Tubular occlusion
|
5.2.1 Strontium-Containing Toothpastes
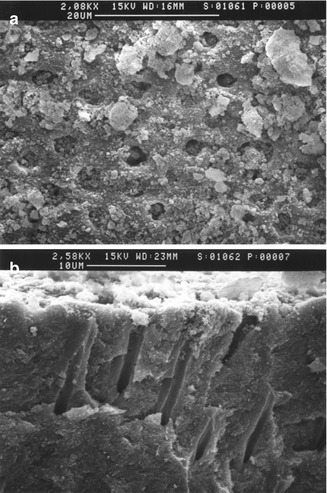
Strontium chloride has been claimed to act as both a protein precipitant and a tubule-occluding agent (Cohen 1961; Skurnik 1963; Blitzer 1967; Gedalia et al. 1978; Uchida et al. 1980). Gutentag (1965) however also demonstrated that strontium may stabilise excitable neural membranes by modifying their permeability to sodium and potassium. Several investigators have shown that strontium ions may be deposited as an insoluble barrier, possibly a calcium strontium–hydroxyapatite complex, at the dentin tubule openings (Pawlowska 1956; Ross 1961; Blitzer 1967; Gedalia et al. 1978). Kun (1976) however demonstrated in vitro that a topical application of concentrated strontium chloride solution produced a continuous deposit on the dentin surface as well as a degree of penetration into the dentin tubules. Furthermore he proposed as a result of evidence from the electron probe microanalysis and X-ray diffraction studies that the fundamental mechanism of the formation of strontium deposits was an exchange with the calcium of the dentin, resulting in recrystallisation in the form of strontium apatite. Evidence from other in vitro studies (Greenhill and Pashley 1981; Mostafa et al. 1983; Pashley et al. 1984; Addy et al. 1991), however, would appear to suggest that these results were attributable not to active ingredient per se but to the abrasive component(s) of a toothpaste which may contribute to the formation of a smear layer and to some degree occlude or block the exposed dentin tubule opening (Mordan et al. 2002) (Fig. 5.2a, b).
5.2.2 Selected Calcium Compounds
5.2.2.1 Casein Phosphopeptide–Amorphous Calcium Phosphate (CPP–ACP)
Toothpastes containing Casein Phosphopeptide–Amorphous Calcium Phosphate (CPP–ACP) were primarily developed for anticaries and remineralisation strategies rather than for the treatment of DH per se. According to Reynolds (1998), the CPP component binds to surfaces in the oral environment such as plaque, bacteria and soft tissue providing a bioavailable Ca and PO4 at the surface of the tooth (e.g. enamel) without any precipitation. The ACP is subsequently released from the dental plaque during acidic challenges. The stabilisation of ACP component by the CPP ensures the delivery of both Ca and PO4 ions onto the enamel surface for remineralisation. Both in vitro and in vivo studies have demonstrated that calcium phosphate preparations deposit a mineral precipitate on to the dentin surface, block dentin tubules and reduce dentin permeability in the dentin disc model and DH in patients (Ebisu 2002; Suge et al. 2002; Cherng et al. 2004; Geiger et al. 2003; Azarpazhooh and Limeback 2008; Charig et al. 2009; Gandolfi et al. 2010; Walsh 2010) (Table 5.1a).
5.2.2.2 Calcium Carbonate and Arginine (Colgate Pro-Argin™)
Kleinberg (2002) suggested at physiological pH the positively charged arginine in the arginine/insoluble calcium carbonate compound combination binds to the negatively charged dentin surface enabling a calcium-rich mineral layer into the open (exposed) dentin tubule to act as an effective plug or tubular occludent. Initial laboratory (in vitro) evidence appears to support this in that the product does occlude the dentin tubules and effectively block fluid flow and is resistant to an acid challenge (Petrou et al. 2009), and subsequent clinical studies evidence appears to support its efficacy as a desensitiser (Ayad et al. 2009; Docimo et al. 2009; Hamlin et al. 2009; Nathoo et al. 2009; Schiff et al. 2009a, b, 2011; Que et al. 2010; Cummins 2011) (Table 5.1a). Recent systematic reviews by Sharif et al. (2013) and Yan et al. (2013) have also indicated that there are clinical benefits for using Pro-Argin™ toothpastes in reducing DH; however both these investigators raised concerns regarding the quality of the conducted studies and recommended that further well-designed studies should be conducted to determine the efficacy of the product in reducing DH.
5.2.2.3 Bioactive Glasses
Bioactive glasses (calcium sodium phosphosilicate), for example, NovaMin® (developed by NovaMin Technology Inc., Alachua, FL, USA) based on the original 45S5 Bioglass® formulation by Larry Hench (US Biomaterials Corp., Jacksonville, FL, USA, now GSK) (Hench 2006), have been incorporated into toothpastes for the treatment of DH. The proposed mode of action is by the precipitating of hydroxycarbonate apatite (HCA) onto the dentin surface and subsequently occluding the dentin tubules (Litkowski et al. 1998; Gillam et al. 2002; Tai et al. 2006; Vollenweider et al. 2007; Burwell 2006; Burwell et al. 2009; Wang et al. 2010; Pradeep and Sharma 2010; Mneimne et al. 2011) (Table 5.1a).
One advantage of the precipitated HCA layer is that it is chemically and structurally similar to natural enamel and dentin (Burwell 2006). A recent randomised double-blind controlled trial, by Orsini et al. (2010), compared the clinical efficacy of a new toothpaste containing (HCA) nanocrystals and a sodium fluoride/potassium nitrate toothpaste and concluded that a new novel toothpaste formulation containing zinc-HCA nanocrystals significantly reduced DH after 4 and 8 weeks. There have however been concerns over the long-term durability of HCA in the oral environment, and it has been postulated that the formation of fluorapatite (FAp) rather than HCA is preferable, since this layer may be more resistant to acid attack and would therefore dissolve less readily when teeth are exposed to acidic conditions (e.g. during consumption of fruit juice and carbonated beverages). It has been recently demonstrated that fluoride-containing bioactive glasses form FAp rather than HCA in physiological solutions (Brauer et al. 2010).
5.2.2.4 Hydroxyapatite-Based Toothpastes
According to Hill et al. (2012), hydroxyapatite-based toothpastes have been widely used in China and the Far East (Park et al. 2005; Kang et al. 2009; Kim et al. 2009; Yuan et al. 2012). The published literature on hydroxyapatite toothpastes however is mainly in non-English journals and may, therefore, not be readily available in an English translation format (Park et al. 2005; Kang et al. 2009; Kim et al. 2009; Yuan et al. 2012). More recently, the commercial emphasis has focused on the use of nanocrystalline hydroxyapatite in toothpastes for desensitising and remineralising strategies (Rimondini et al. 2007; Orsini et al. 2010; Tschoppe et al. 2011). The proposed mechanism of action for hydroxyapatite-containing toothpastes is blocking the dentin tubules (Rimondini et al. 2007; Hill et al. 2012; Yuan et al. 2012) (Table 5.1a).
5.2.3 Selected Fluoride Formulations
Fluoride was first proposed as a desensitising agent in 1941 by Lukomsky (1941) and has subsequently used in toothpastes, gels, mouth rinses and varnishes (Orchardson and Gillam 2006). Sodium fluoride and stannous fluoride have been shown to reduce DH (Morris et al. 1999), and amine fluoride has also been incorporated into dentifrices although there is currently limited published data to support its use. Stannous fluoride (SnF2) in a 0.4 % glycerin gel has also been reported to be effective in reducing DH (Miller et al. 1969) although this formulation and the use of SnF2 may be problematic for a number of reasons, for example, (1) when placed in an aqueous environment, it appears to undergo hydrolysis and precipitates out of solution (Miller et al. 1969) hence the incorporation into a gel and (2) poor taste and staining characteristics. More recently investigators have demonstrated that a reformulated toothpaste containing stannous fluoride with a novel 0.454 % stabilised stannous fluoride formulation containing sodium hexametaphosphate (SHMP) was effective in reducing DH during an 8-week treatment compared to a sodium fluoride toothpaste as a control (Schiff et al. 2005, 2006; Day et al. 2010; Einwag et al. 2010; cited by Ni et al. 2010). According to Greenhill and Pashley (1981), fluorides decrease the permeability of dentine in vitro possibly by the precipitation of insoluble calcium fluoride within the tubules. However the exact mechanism whereby fluoride reduces DH is unknown. Fluoride incorporation increases the resistance of dentin to decalcification (Furseth 1970) and reduces its solubility (Sandoval and Shannon 1969) as fluorapatite is more resistant to acid attack(s) than hydroxyapatite. Sodium monofluorophosphate has also been previously investigated as a toothpaste ingredient with desensitising effects by Hazen et al. (1968) and by Addy et al. (1987) who reported on its clinical effectiveness in combination with strontium acetate. Recently several investigators have reported on the clinical efficacy of a combined sodium monofluorophosphate/strontium acetate formulation for the treatment of DH (Mason et al. 2010; Hughes et al. 2010) (Table 5.1b). Higher fluoride concentration toothpastes containing 2,800/5,000 ppm sodium fluoride (Colgate Duraphat) have also been advocated for prevention of dental caries and as such may be of potential benefit in the treatment of root caries and DH.
One however should not ignore that natural desensitisation of dentin (both internally and externally) may occur irrespective of whatever treatment is provided by the clinician to the patient. For example, Orchardson and Gillam (2006) have suggested that there may be natural desensitising of dentin through precipitation of salivary proteins, toothpaste ingredients, etc., forming a smear layer which may occlude the dentin tubules or remineralisation (Pashley 1992a; Kawasaki et al. 2001) as well as the formation of both intra-tubular dentin and secondary/tertiary dentin over time (Addy and Dowell 1983) (Fig. 5.1).
5.3 Nerve Desensitisation and Noci0ception
As indicated earlier, the hydrodynamic theory (Brännström 1963) is generally considered to be the mechanism of choice, and as with dentin blocking agents, this theory appears to explain how nerve desensitisation may occur following the application of potassium-containing products (e.g. toothpastes gels, mouth rinses).
Several investigators (Greenhill and Pashley 1981; Pashley et al. 1984) however failed to observe any effect of potassium nitrate (either as a 30 % solution or 5 % toothpaste) in terms of a reduction in dentin fluid flow (dentin permeability) in the in vitro dentin disc model. In other words, these investigators were unable to demonstrate whether potassium nitrate reduced DH by blocking the dentin tubules and were therefore unable to determine the exact mechanism of action of potassium-containing toothpastes which were reported to be clinically effective when treating DH. These investigators however did not rule out the possibility that these agents may desensitise dentin via neural effects unrelated to hydrodynamic mechanisms.
In order to ascertain the precise mechanism for action for potassium, several investigators utilised a neurophysiological animal (cat) model which involved deep-cut cavity preparations with a very thin slice of dentin between the exposed dentin surface and the pulp (Kim 1986; Markowitz and Kim 1985, 1990; Markowitz et al. 1991). These investigators subsequently demonstrated that when using large molar concentrations of various divalent cation solutions (including potassium) applied to the dentin surface, both intradental nerve activity and sensory nerve activity were reduced. It was also evident from these studies that the important chemical moiety of potassium nitrate was the potassium salt and not the nitrate anion as previously believed. Furthermore potassium appeared to be the more effective desensitising agent compared to the other solutions tested irrespective of which combination of anion was used. On the basis of these observations, these investigators proposed that the mode of potassium desensitisation was through raising the intra-tubular potassium (K+) concentration which would render the intradental nerves less excitable to any further stimulation by depolarisation of the nerve fibre membrane. Initially this increase in the potassium ion content elicits an increased number of action potentials, after the initial depolarisation; however the nerve fibre(s) cannot depolarise due to the maintained high levels of extracellular potassium ion content and as a consequence a sustained depolarised state occurs (axonal accommodation).
The interpretation based on the investigation by Kim and co-workers (1985, 1986, 1990, 1991 see above) has however been criticised by Sena (1990) who suggested that as a result of the deep-cut cavity preparations in the cat, the applied potassium ion only had a short distance to traverse the length of the dentin tubule to exert its effect to desensitise the nerve. In the normal clinical situation (in intact human teeth), however, the incoming potassium ion (e.g. if applied in a toothpaste product on the exposed cervical dentin) would have to overcome the opposing pulpal pressure that produces an outward flow of dentin fluid. Such an outward flow may therefore prevent the inward diffusion of substances from the oral cavity. Currently it is important to note that this proposed mechanism was based on animals and has not been confirmed for human dentin (Orchardson and Gillam 2000) (Table 5.2). For example if the desensitising effects of potassium are due to action potential inactivation, one might expect as Orchardson and Gillam (2000) suggested that the patient would experience a transient pain when a potassium-containing toothpaste is applied to the exposed dentin surface. This phenomenon has not been reported for toothpastes in humans. It may also be of note in this context, however, to reconsider the work by Anderson and co-workers (1958, 1962a, b) who postulated that if dentin was directly innervated, then chemical stimuli to the exposed dentin surface should cause a patient discomfort. Application of algogenic (pain-inducing) substances such as potassium chloride, acetylcholine and histamine, however, failed to elicit a response. By way of contrast when these substances were applied directly to exposed pulpal tissue, an immediate response was elicited (Anderson and Naylor 1962; Anderson 1968, 1972). This observation may therefore be of interest when ascertaining the precise mode of action of potassium-containing preparations.
Table 5.2
Characteristics of selected nerve depolarising toothpastes
|
Product
|
Composition
|
Proposed mode of action
|
|---|---|---|
|
Sensodyne
|
Potassium nitrate, sodium fluoride (NaF)
|
Hydrated toothpaste technology. Evidence of a desensitising action based on historical animal studies. No evidence of tubular occlusion when potassium ions were tested in vitro. Evidence from the published literature suggests that potassium-containing toothpastes are effective in reducing dentin hypersensitivity although there is no evidence to suggest that it is by nerve depolarisation. Recent clinical study has reported that there is a transient depolarising effect when potassium ions are applied on exposed dentin
|
|
Colgate
|
Potassium nitrate, sodium monofluorophosphate (MFP)
|
|
|
Crest
|
Potassium sodium fluoride (NaF)
|
|
|
Sensodyne
|
Potassium chloride sodium fluoride (NaF)
|
|
|
Colgate
|
Potassium citrate sodium monofluorophosphate (MFP)
|
Several investigators have however attempted to explain the role of potassium diffusion across dentin (Stead et al. 1996; McCormack and Davies 1996).
(a)
Mathematical Model of Potassium Ion Diffusion
In order to ascertain whether the potassium ion could diffuse down the dentin tubule, Stead et al. (1996) proposed a mathematical model of potassium ion diffusion which incorporated a number of variables, for example, dentin thickness, tubule diameter, time, diffusion gradient, outward fluid flow, the constituents of dentin fluid (molecule size), permeability of the odontoblast layer and the concentration of potassium (based on 5 % potassium in toothpastes).
According to these investigators, the application of potassium-containing preparations to the exposed dentin may increase potassium ions at the inner ends of the dentinal tubules to levels sufficient to inactivate intradental nerves; however, the localised increase in potassium ions may only be transient, and the concentration change will also be reduced by conditions that increase the tubular fluid flow velocity or the permeability of the barrier between the tubule and the pulp. The prediction from this model regarding nature of the transient effect of the potassium ion on nerve inactivation may be of interest particularly in the light of the results from the clinical studies by Ajcharanukul et al. (2007, 2011, 2012). These investigators utilised a cut cavity preparation approach based on the animal model in human subjects and demonstrated that potassium salts had a transient desensitising effect as predicted by Stead et al. (1996). However one of the conclusions from these studies was that the hydrodynamic mechanism responsible for responses to stimulation of dentin in humans has different properties from those demonstrated in the cat and may not necessarily be mediated by a hydrodynamic mechanism.
(b)
The Role of Nitric Oxide as a Secondary Messenger
One of the problems with the mathematical model of potassium diffusion as proposed by Stead et al. (1996) was the various constraints to the diffusion of the potassium ion along the entire length of the dentin tubule (Orchardson and Gillam 2000). An alternative mechanism for potassium ion-mediated desensitisation was proposed by McCormack and Davies (1996). These investigators suggested that the potassium ion could evoke a novel synthesis of a mobile secondary messenger (nitric oxide) within dentin and the dental pulp. The proposed hypothesis is that the potassium ion may act on the odontoblast process to release nitric oxide (in the dental pulp) which in turn produces an analgesic effect by modulating nociceptive input through downregulation of sensitised nociceptors. Although this hypothesis may provide a plausible explanation for the role of the potassium ion in the treatment of DH there does not appear to be any supporting evidence from the published literature (Orchardson and Gillam 2000; Jackson 2000).
According to Orchardson and Gillam (2000), there appears to be no convincing evidence that desensitising preparations based on potassium chloride, nitrate and citrate act in the manner proposed. It is possible that any desensitising effects may be due to constituents other than the potassium salts. Although there is some evidence that toothpastes containing potassium ions are more effective than minus-active preparations in reducing dentin hypersensitivity, the potassium-containing preparations are not always superior to controls such as sodium monofluorophosphate. Furthermore while a number of studies included in the Orchardson and Gillam (2000) review reported that potassium-containing salts were significantly better than the inactive (placebo) controls, a number of these studies did report an appreciable reduction in dentin hypersensitivity with the supposedly ‘inactive’ controls. One of the problems, in evaluating the various studies was the reported variation(s) in the extent of the ‘control/placebo’ response which may have accounted for most of the disparities between trial outcomes (Jackson 2000; Cummins 2009, 2010). A previous published systematic review by Poulsen et al. (2006) included six studies in the meta-analysis and concluded that there was no clear evidence available in the published literature for the support of potassium-containing toothpastes for the relief of DH. More recent reviews by Pol et al. (2010) and Karim and Gillam (2013) also highlighted the lack of data on the efficacy of potassium salts in reducing dentin hypersensitivity.
The use of topical guanethidine (1 % guanethidine solution (Ismelin, Ciba-Geigy, UK)) as a desensitiser has also been advocated although there are only two published studies by Hannington-Kiff and Dunne (1993) and Dunne and Hannington-Kiff (1993). These investigators proposed that topically applied guanethidine affects the anti-noradrenergic mechanisms in the teeth.
5.4 Placebo Effect
Both placebo and nocebo effects have been documented in the published literature and may impact on the results from studies evaluating the efficacy of a drug. The term ‘nocebo’ comes from the Latin ‘noceo’, to harm, and means ‘I shall harm’, whereas the term ‘placebo’ means ‘I shall please’ (Definition of placebo 2013: http://www.medterms.com). A negative placebo effect may occur during a clinical study where patients participating in the study experience adverse side effects unrelated to the specific pharmacological action of the drug that they are taking. The nocebo effect may be associated with a subject’s prior expectations of adverse effects from treatment as well as with conditioning in which the subject learns from prior experiences to associate a medication with certain somatic symptoms (Definition of nocebo effect 2013: http://www.medterms.com). The placebo or placebo effect has been defined in the following manner:
(a)
A substance containing no medication and prescribed or given to reinforce a patient’s expectation to get well.
(b)
An inactive substance or preparation used as a control in an experiment or test to determine the effectiveness of a medicinal drug.
(c)
An active placebo – a placebo used in experimental tests of a drug that has noticeable side effects; ‘an active placebo mimics the side effects of the experimental drug’ (Definition of active placebo 2013: www.thefreedictionary.com ).
According to Oken (2008), the interaction between the clinician and the subject during a clinical study may have an impact on outcomes independent of any specific treatment. For example, ‘expectancy’ may be affected by the personal history of subject–clinician interactions and shared experiences of the subject and clinician. Several investigators have also suggested that any placebo effects during a study may also be influenced by the number of subject–clinician interactions (Ilnyckyj et al. 1997; Paternak and Zimmerman 2007 cited by Oken 2008). There may also be other non-specific benefits from this interaction during a clinical study, for example, stress reduction, decreased anxiety or improvement of mood of the subject. According to Oken (2008), some clinicians are perceived to be better clinicians than others as a result of their personality or interaction style. These factors may therefore have profound effects in clinical studies, in particular pain-type studies, for example, a dentin hypersensitivity study, designed to evaluate the efficacy of various desensitising agents.
For example, a number of published studies evaluating desensitising toothpastes have demonstrated improvement in symptoms ranging from 30 to 80 % reduction in sensitivity when comparing test toothpastes to other toothpastes and placebo controls (Clark and Troullos 1990). The results from these studies are however somewhat conflicting and difficult to interpret, due in part to different methodologies and patient selection criteria. One of the main inherent problems in conducting clinical studies designed to assess the efficacy of desensitising products is the interference of placebo and/or Hawthorne effects that may introduce a degree of bias into the study (Gillam 1997, 2011; Addy et al. 2007). Several investigators have suggested that the utilisation of a double-blind placebo-controlled study is one possible way of resolving this particular bias, although such effects cannot be completely eliminated (Jeffcoat 1993; Holland et al. 1997; ADA Acceptance Program Guidelines 2012). For example, several investigators have reported that this effect can be as high as 40 % (Curro et al. 2000; West et al. 1997). Other investigators have also alluded to this effect in their published studies (Gillam et al. 1996, 1997a; Pearce et al. 1994; Chesters et al. 1992), but to what extent the placebo effect complicates the interpretation of the results of the study is difficult to predict. It should however be noted that according to Curro et al. (2000) the placebo effect observed in dentin hypersensitivity studies is not too dissimilar to those reported in other medical and dental therapeutic studies. For example, a review of 15 post-operative pain studies by Beecher (1955) cited by Curro et al. (2000) concluded that on average symptoms were satisfactorily relieved by the placebo medication in 35 % of the patients (the placebo response range of 15–58 %). According to Hróbjartsson and Gøtzsche (2001) in a systematic review detailing 27 trials involving the treatment of pain, the placebo had a beneficial effect, as indicated by a reduction in the intensity of pain of 6.5 mm on a 100-mm visual-analogue scale. If the magnitude of the placebo effect is reproduced in a clinical study, this may well confound any effects of efficacy of the active product. These and other confounding factors, for example, a random variation in patient symptoms over time (regression to the mean/mode, conditioning effects during the study, small sample size) affecting dentin hypersensitivity studies may also be complicated by the lack of universally acceptable positive and negative controls used in equivalence and superiority studies (Gillam 2011). A further problem that may confound determining the efficacy of these desensitising products is that the clinical efficacy of these products may be at the lower end of the therapeutic range (Addy et al. 2007). Curro et al. (2000) also suggested that subjects with chronic conditions such as dentin hypersensitivity typically have episodic or fluctuating symptoms and any potential change in these symptoms over time in a clinical study may be one of improvement (the so-called expectancy effect). A patient’s expectancy of improvement may therefore influence outcomes as much as some active interventions, and this effect may be greater for novel interventions and for procedures (Oken 2008). It may therefore be suggested that the clinical study duration should be of a suitable duration (e.g. at least 6 weeks) as to minimise any ‘placebo effects’.
It is important however to acknowledge that the amount of time required for a particular desensitising agent to achieve clinical effectiveness may be affected by several factors, including (a) variations in the motivation of individual patients and their ability to apply the product as intended and (b) the nature of the test agents and their likely mode of action. These factors may therefore dictate the design, nature and duration of any proposed clinical study.
5.5 Restorative Approaches
There are a number of restorative approaches for the treatment of DH that are provided for patients with localised moderate to severe DH which require immediate palliative alleviation (Orchardson and Gillam 2006). As indicated in Chap. 6, these desensitising agents may be classified on the basis of (1) whether products do not polymerise (varnishes/precipitants/primers containing HEMA), (2) whether they undergo setting or polymerisation reactions (conventional glass ionomer cements, or resin-reinforced glass [ionomers/compomers; adhesive resin primers; adhesive resin bonding systems), (3) the use of mouthguards, (4) iontophoresis combined with fluoride pastes or solutions and (5) lasers (Pashley 2000). Examples of these products are resins, varnishes, primers, dentine bonding agents and glass ionomer cements which contain fluoride, aluminium, potassium or ferric oxalates; silica or calcium-containing materials; and protein precipitants to decrease dentin permeability or block the fluid movement through dentin (Tables 5.3 and 5.4). Other miscellaneous treatment approaches have also been recommended, for example, occlusal adjustment associated with cervical abfraction lesion (Coleman et al. 2003), crown restorations, root coverage surgery, pulp extirpation, extraction (Ong and Strahan 1989), homoeopathic remedies (Plantago) (www.hpathy.com), propolis (Mahmound et al. 1999) and hypnosis (Starr et al. 1989; Eitner et al. 2010). It should however be acknowledged that some of these restorative procedures may also initiate post-operative sensitivity, for example, crown preparations, restorations, restorative materials, nonsurgical (scaling) and surgical procedures and sensitivity from bleaching or whitening procedures. One of the problems, however, when recommending or evaluating these restorative approaches for the treatment of dentin hypersensitivity is that dental professionals not only appear to be uncertain as to the most successful way in which to manage dentin hypersensitivity but also express a level of dissatisfaction with the various products and techniques available (Cunha-Cruz et al. 2010).
Table 5.3
Selected dentin desensitising solutions and products tested in clinical trials
|
Type, chemical/concentration
|
Product and clinical support
|
|---|---|
|
Fluorides
|
|
|
Sodium fluoride, stannous fluoride, hydrogen fluoride
|
|
|
Potassium nitrate
|
|
|
1–15 % solutions
|
Hodash (1974)
|
|
5, 10 % in gel
|
Frechosa et al. (2003)
|
|
Oxalate
|
|
|
3 % potassium oxalate
|
Protect, Sunstar Butler, Chicago, IL, USA Camps and Pashley (2003)
|
|
3 % potassium oxalate
|
Oxa-gel, Art-dent Ltda, Araraquara, SP, Brazil Pillon et al. (2004)
|
|
6.8 % ferric oxalate
|
Sensodyne Sealant, GSK, Jersey City NJ, USA Gillam et al. (2004)
|
|
Calcium phosphates
|
|
|
1.5M calcium chloride + 1.0M potassium oxalate
|
Geiger et al. (2003)
|
|
D/Sense 2 (Centrix Direct)
|
Kolker et al. (2002) (in vitro)
|
|
Quell Desensitizer (Pentron Clinical Technologies)
|
|
Table 5.4
Selected professionally applied dentin desensitisers tested in clinical trials
|
Type
|
Product and clinical support
|
|---|---|
|
Fluoride varnish
|
|
|
Fluoline, PD Dental, Altenwalde, Germany (Duran and Sengun 2004)
|
|
|
Duraflor (Pharmascience) (Merika et al. 2006)
|
|
|
Fluor Protector (Ivoclar Vivadent)/AllSolutions Fluoride Varnish (Dentsply) (Ritter et al. 2006)
|
|
|
Oxalic acid + resin
|
MS Coat, Sun Medical Co, Shiga, Japan (Prati et al. 2001)
|
|
Pain-Free, Parkell Co, Farmingdale, NY, USA (Morris et al. 1999)
|
|
|
Sealants, primers
|
|
|
Dentin Protector, Vivadent, Germany (Schwarz et al. 2002)
|
|
|
Gluma Alternate, Heraeus Kulzer, Wehrheim, Germany (Dondi dall’Orologio et al. 1999)
|
|
|
Hemaseal and Cide (Germiphene)/HurriSeal Dentin Desensitizer (Beutlich Pharm.) (Kolker et al. 2002)
|
|
|
One-Step (Bisco, USA) (Kakaboura et al. 2005)
|
|
|
Prime and Bond 2.1, Dentsply Caulk, Milford, DE, USA (Swift et al. 2001)
|
|
|
Etch + primer
|
Scotchbond, 3M Dental Products, St Paul, MN, USA (Ferrari et al. 1999)
|
|
Systemp.desensitizer, Ivoclar Vivadent, Schaan, Liechtenstein (Stewardson et al. 2004)
|
|
|
Etch + primer + adhesive
|
Scotchbond Multi-Purpose Adhesive, 3M Dental Products, St Paul, MN, USA (Dondi dall’Orologio et al. 1999)
|
|
Primer + adhesive
|
SE Bond, Kuraray, Okayama, Japan (Duran and Sengun 2004)
|
|
Glass ionomer cements
|
|
|
Fuji VII (GC) (Polderman et al. 2007)
|
1.
Selected Non-polymerising Products
These products include varnishes/precipitants/primers containing HEMA.
Historically varnishes and cavity liners such as Copalite have been recommended for the treatment of dentin hypersensitivity (Wycoff 1982), although most of these varnishes appear to provide inadequate insulation against thermal conduction under restorative materials (Voth et al. 1966). Varnishes such as copal varnishes (copal resin in an ether solution) were shown to be incompatible with the resin-based restorations due to their effect on the polymerisation process (Tjan and Chan 1987). As a result, a number of resin-compatible cavity varnishes, for example, Univar/Uniseal/Microjoin (Sci Pharm Duarte, Ca, USA) were introduced and evaluated on their ability to block dentin tubules (Tjan and Chan 1987; Tjan et al. 1987). Fluoride varnishes such as Duraphat® (Colgate Oral Pharmaceuticals), Dentinbloc (Colgate Oral Pharmaceuticals), Bifluorid 12 (VOCO GmbH), Isodan® (Septodont), Shellac F Cervitec® or Fluor Protector (Ivoclar Vivadent) have also been previously evaluated for the treatment of dentin hypersensitivity (Collaert et al. 1991; Thrash et al. 1992; Kielbassa et al. 1997; Gaffar 1999; Morris et al. 1999; Panduric et al. 2001; Merika et al. 2006; Ritter et al. 2006; Hoang-Dao et al. 2009; Bhandary and Hegde 2012). According to Ritter et al. (2006), the topical application of fluoride varnishes was thought to create a barrier by the precipitation of CaF2 onto the exposed dentin surface which, in turn may occlude the dentin tubules thereby reducing dentin permeability and, as a consequence, DH. From a practical viewpoint, the application of a fluoride varnish may be useful in identifying whether a patient has DH during the diagnosis examination in order to rule out any other dental cause. The application of fluoride varnishes may also be incorporated in a stepwise management programme where non-invasive procedures are undertaken, and depending on whether the problem has been resolved or not, the clinician may either proceed to provide additional applications of the varnish or opt to provide a more invasive procedure (Orchardson and Gillam 2006). Other treatment approaches include the application of Hema-containing primers, for example, Gluma (5 % glutaraldehyde primer and 35 % hydroxyethyl methacrylate), calcium hydroxide and oxalate varnishes (Pashley 2000; Orchardson and Gillam 2006) (Tables 5.3 and 5.4). The efficacy of Hema-containing primers in treating DH has been evaluated in a number of clinical studies. For example, Felton et al. (1991) applied the primer to the facial surfaces of crown preparations in 20 patients and reported that in response to air, tactile and osmotic stimuli, DH was significantly reduced after 14 days compared to the control group. Other investigators (Dondi dall’ Orologio and Malferrari 1993, Dondi dall’ Orologio et al. 1999, 2002; Duran and Sengun 2004) reported similar successful results when using the primer on exposed dentin. However a study by de Assis et al. (2006) in periodontal patients with hypersensitive teeth failed to demonstrate any efficacy with Gluma Desensitizer® compared to the control group. A more recent study by Mehmood et al. (2011) compared Gluma Desensitizer® with Duraphat® in 196 patients with non-carious cervical lesions. They conclude that Gluma Desensitizer® significantly reduced DH compared to the Duraphat® varnish. A 6-month study by Aranha et al. (2009) evaluated Gluma Desensitizer® with four other products or therapies (Seal and Protect, OXA GEL, fluoride and low-intensity laser treatment). They concluded that although both Gluma Desensitizer® and Seal and Protect had an immediate effect in reducing DH, all therapies demonstrated lower sensitivity scores at the 6-month evaluation point. The proposed mechanism of blocking the tubules with HEMA-containing primers may be a result of the glutaraldehyde component reacting with the albumin within the dentin fluid by protein precipitation; this in turn may reduce the outward fluid flow and as a consequence reduce DH (Pashley 2000).
The application of oxalate-containing solutions has also been evaluated for treating DH (Muzzin and Johnson 1989; Salvato et al. 1990; Kerns et al. 1991; Morris et al. 1999; Gillam et al. 1997, 2004; Pashley et al. 2001; Camps and Pashley 2003; Tay et al. 2003; Pillon et al. 2004; Pamir et al. 2007) although according to Pashley (2000) and Orchardson and Gillam (2000), the clinical evidence is somewhat inconclusive. In this context it is of interest that despite this reservation on the efficacy of these products, 40 % of practising dentists in the USA reported using oxalate preparations in order to treat DH (Cunha-Cruz et al. 2010). A systematic review by Cunha-Cruz et al. (2011) concluded that many of the oxalate products that were included for evaluation in the review were no better than the placebo controls with the possible exception of a 3 % monohydrogen monopotassium oxalate solution. These investigators concluded that the current evidence did not support recommending using oxalates for the treatment of DH. The mechanism by which oxalate products block the dentin tubules has been demonstrated by a number of investigators (Greenhill and Pashley 1981; Gillam et al. 2001; Yiu et al. 2005). According to Yiu et al. (2005) following the application of the oxalate solution on the depletion of calcium ions from the surface dentin forces the oxalate ions to diffuse further down into the dentin tubule and react to form insoluble calcium oxalate crystals. This reaction results in a subsurface tubular occlusion which will reduce fluid flow (dentin permeability) within the dentin tubules.
According to Pashley (2000), the use of calcium hydroxide paste has been applied for the treatment of DH. For example, Green et al. (1997) applied a 5-min treatment of calcium hydroxide on hypersensitive root surfaces and reported that in response to thermal and mechanical stimuli, DH was reduced for the duration of the 3-month study. Wolfart et al. (2004) also compared a calcium hydroxide solution with a glutaraldehyde-based dentin primer in 36 patients undergoing crown preparations and evaluated over a 30-month period. Although the investigators reported that a calcium hydroxide solution may be useful in treating DH, there were no reported differences between the two products. According to Ling and Gillam (1996) citing McFall (1986), calcium hydroxide blocks the dentin tubules by a deposition of calcium ions that bind to free protein radicals and increasing the remineralisation of the exposed dentin. It was claimed that the initial application was successful for 80–90 % of the time, but this effect rapidly diminished and frequent reapplication was required. Pashley et al. (1986) applied a calcium hydroxide paste to human dentin in vitro in order to determine its effects on dentin permeability. The results indicated that although the paste reduced dentin permeability in both smear layer and non-smear layer samples, calcium hydroxide provides little protection to an acid challenge. On the basis of these studies, it would appear that the use of calcium hydroxide on exposed root surfaces may be of limited value.
2.
Selected Products That Undergo Setting or Polymerisation Reactions
These products include conventional glass ionomer cements, or resin-reinforced glass ionomers/compomers; adhesive resin primers; and adhesive resin bonding systems.
The use of conventional glass ionomer cements (GIC) or resin-reinforced glass ionomers/compomers has been recommended for a number of clinical conditions in the oral cavity, for example, as a liner in prepared cavities (Hansen 1992; Tantbirojn et al. 2006; Burrow et al. 2009), fissure sealing (Pardi et al. 2003), cementing orthodontic brackets (Charles 1998), treatment of dentin hypersensitivity (Wycoff 1982), non-carious cervical lesions (Francisconi et al. 2009) and a combined surgical/restorative intervention of gingival recession with associated non-carious cervical lesions (Santamaria et al. 2007
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses