Fig. 2.1
Theories of dental pain. (a) Old theory that pulpal nerves extended out to the DEJ and made the DEJ sensitive. However, careful histology failed to demonstrate nerves at the DEJ. (b) In the 1950s, scientists thought that pulpal nerves synapsed with odontoblasts and that odontoblast processes extended to the DEJ. Careful histology revealed that odontoblast processes do not extend to the DEJ. (c) The hydrodynamic theory of dentin pain is that movements of dentinal fluid in tubules in either direction activate mechanoreceptor nerves in the pulp to cause dentin sensitivity (From Ten Cate (1998), p. 191 with permission)
2.2 Fluid Flow Models of Sensitivity
Our group was the first to report dentinal fluid flow across dog dentin in vivo (Pashley et al. 1981). In that same paper, we reported pulpal tissue pressures in dogs of 15–47 cm H2O and that dentinal fluid flow was driven by pulpal tissue pressure (Fig. 2.2). This was later confirmed in humans in vivo (Ciucchi et al. 1995). In that paper, we reported pulpal tissue pressures of 14–15 cm of water that was similar to pulpal tissue pressure in cats of 16 cm H2O reported by Vongsavan and Matthews (1992). Vongsavan and Matthews actually calculated the velocity of outward movement of dentinal fluid in cat dentin at 1.4 μm s−1. This outward seepage of dentinal fluid flow occurs because the pulpal tissue pressure is 16 cm H2O greater than atmospheric pressure in normal pulps. This slow outward fluid flow is too slow to activate pulpal nerves. Using radioactive 125I, Pashley and Matthews (1993) confirmed that outward directed convective fluid flow could significantly restrict the inward diffusion of small molecules. After Nissan et al. (1995) showed that bacterial endotoxin can diffuse through human dentin, Puapichartdumcong et al. (2005) reported that outwardly directed fluid flow under 15 cm H2O pressure could significantly lower the flux of endotoxin across dentin. Thus, although outward fluid flow reduces inward diffusion, it does not eliminate it.
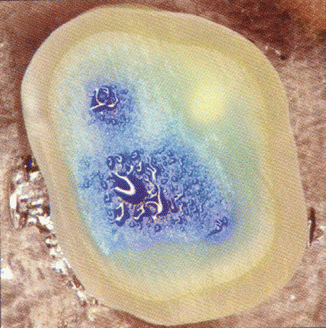

Fig. 2.2
Flattened occlusal surface of a tooth in vitro filled with blue dye that slowly seeps across open dentinal tubules under a pulpal tissue pressure of 20 cm H2O. The tubules over pulp horns are shorter and are located closer together, making these tubules very sensitive (From Pashley and Tay (2012), with permission)
2.3 Effects of Inflammation of the Pulp
During pulpal inflammation, a large number of chemical mediators of inflammation are released, including histamine and serotonin, complement activation, and bradykinin release (Fouad 2012). Many of the mediators make pulpal nerves more sensitive than normal and cause the surrounding fibroblasts to divide more rapidly than normal. Fibroblasts release more nerve growth factor (NGF) that, in turn, causes nerve terminals to sprout and increase their neuropeptide content. This increase in nerve density is thought to be associated with the development of dentin hypersensitivity (Fig. 2.3). A receptive field in dentin is all of the dentinal tubules innervated by a single nerve and its branches. An increased size of receptive fields in inflamed pulps has been reported (Byers and Närhi 1999). Most of the nerves that sprout contain calcitonin gene-related product (CGRP) and substance P (SP). Both neuropeptides are known to vasodilate pulpal blood vessels and increase capillary permeability. These reactions lead to increases in tissue pressure (Heyeraas and Kvinnsland 1992; Berggren and Heyeraas 1999) and in outward fluid flow. This increase in outward fluid flow occurs not because dentin is more hyperconductive, but because of increases in local tissue pressure, the driving force for dentin fluid flow (Pashley 1992). As this fluid flows through narrow tissue spaces between pulpal nerves and dentin, the local shear forces on mechanoreceptors may bring the resting membrane potential of pulpal nerves closer to threshold, making them “hypersensitive” (Fig. 2.4). Working in cats in vivo, Matthews and Vongsavan (1994) reported that application of a negative pressure of −300 mmHg caused outward fluid flow to increase from 1.4 to 25 μm s−1 to fire pulpal mechanoreceptors. They calculated that fluid flow rates >1.5 nl s−1 mm−2 were required to activate intradental nerves in anesthetized cats.
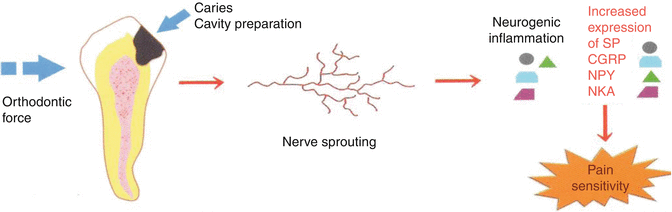
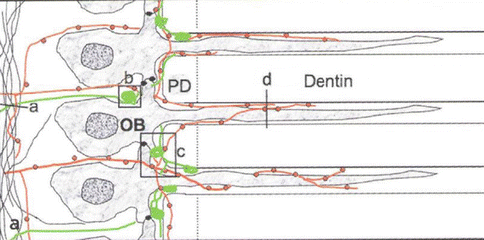

Fig. 2.3
Although this figure shows neuronal responses of the pulpodentin complex to caries, cavity preparation, or orthodontic tooth movement, many authorities believe that hypersensitive pulps undergo the same responses to inflammatory mediators (From Fouad (2012), with permission)

Fig. 2.4
Schematic of odontoblasts extending cellular processes out into deep dentin. Nerves in red (C-fibers) contain peptide neurotransmitters, substance P, and calcitonin gene-related peptide (CGRP). The larger green nerves are A-δ sensory nerves that carry sharp, well-localized pain of dentin sensitivity. OB designates the cell body of an odontoblast; PD designates the presence of predentin interposed between the odontoblast layer on the left, and mineralized dentin on the right (From Byers et al. (2012), p. 136)
2.4 Effects of Inflammation on Sodium Channels in Nerves
Inflammation also modifies the types of sodium channels that are distributed in sensory nerves. Sodium channels can be classified as tetrodotoxin sensitive or tetrodotoxin (TTX) resistant. When nerves are surrounded by inflammatory mediators, they not only sprout, they also upregulate new sodium channels (Nav 1.8 and 1.9) that seem to be more easily activated (Fig. 2.5) than is seen in the absence of inflammation (Renton et al. 2005; Wells et al. 2007; Luo et al. 2008; Warren et al. 2008; Henry et al. 2009). Thus, in clinical cases of dentin hypersensitivity, if patients cannot successfully remove bacterial plaque on exposed hypersensitive dentin, that dentin may remain hypersensitive until some tubule occluding agent can reduce in inward flux of plaque products. These bacterial products sustain the localized pulpal inflammation, which makes pulpal nerves hypersensitive to hydrodynamic stimuli that might not normally cause pain.
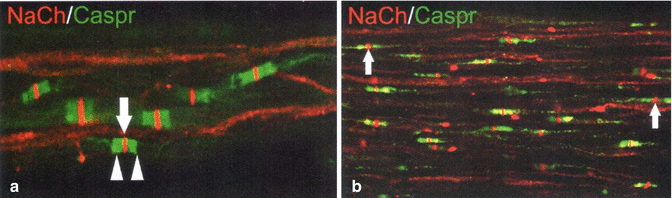

Fig. 2.5
(a) Confocal micrographs of sodium channels (red) Nav 1.7 and Nav 1.8 in pulpal axons and Caspr (green) that identify nodes of Ranvier in mylinated nerves in normal pulp. (b) On the right panel are shown those same neural proteins in inflammed pulps. Note the presence of more nerves in painful pulps (From Byers et al. (2012), p. 124)
Partial or complete tubule occlusion is an important part of treating dentin hypersensitivity, but plaque control is also very important. Tubule occluding agents may be more effective clinically if they include antibacterial agents that can control plaque.
When dentin is freshly exposed, it is very sensitive, even when the underlying pulp is healthy. However, if the freshly exposed dentin becomes covered with bacterial plaque, the tooth becomes hypersensitive when the pulps show signs of local inflammation beneath the exposed dentinal tubules (Lundy and Stanley 1969). What is in bacterial plaque that causes exposed dentin to become hypersensitive?
2.5 Effects of Bacterial Products on the Pulp
In 1962, Brännström recruited teenaged children whose premolars were scheduled for extraction for their orthodontic treatment. Under local anesthesia, he ground through the enamel of those premolars into the mid-coronal dentin to expose 5–10 mm2 of dentin covered by smear layer (Fig. 2.6a). He tested the initial sensitivity of the exposed dentin to air blasts and tactile probing and recorded their pain responses. He left the drilled cavities unfilled and exposed to saliva and oral bacteria. One week later, he had them return for rescoring of dentin sensitivity (Fig. 2.6b). He found that sensitivity had increased a great deal due, in part, to the loss of the smear layer. When the teeth were extracted for orthodontic treatment, and the pulps were examined histologically, he found that the pulpal surface of cut, exposed tubules that were allowed to dry in air, showed aspiration of some odontoblast nuclei into tubules. When dentin was exposed to saliva for a week, the pulps became infiltrated with acute inflammatory cells (Figs. 2.7a, b).
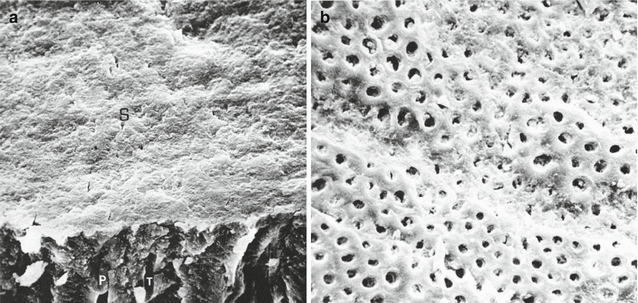
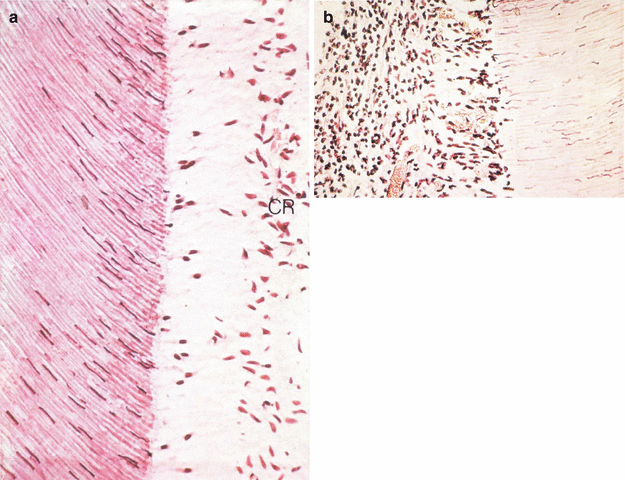

Fig. 2.6
(a, b) Brännström prepared shallow cavities in orthodontic premolars scheduled for extraction. The teeth were not restored but were left open to the oral cavity for up to 7 days. Sensitivity was scored immediately and after 1 week. The teeth were extracted and examined histologically and by SEM. On the left, the freshly prepared cavity dentin was covered with a smear layer. On the right, the smear layer dissolved from the exposed dentin after 1 week. Note how open are the tubules (From Figs. 14 and 15, Brännström (1981), with permission)

Fig. 2.7
(a) This tooth was allowed to air dry for 4 min and became painful after 20 s. Note the odontoblast layer has disappeared from beneath the dentin. Odontoblast nuclei are seen up inside the tubules, but no inflammation has occurred yet. One can easily identify the cell-free zone and the cell-rich (CR) zone (From Figs. 14 and 15, Brännström 1981, with permission). (b) This tooth’s class V cavity was exposed to oral fluids for 1 week. The dentin was very painful. The pulp was heavily infiltrated with acute inflammatory cells. Pulpal capillaries were disrupted and venules were dilated (From Figs. 14 and 15, Brännström (1981), with permission)
We now know that the ground dentin was covered by a smear layer that became covered with bacterial plaque within days. This biofilm created sufficient lactic acid to dissolve the smear layer and smear plugs (Kerns et al. 1991) within 1 week so that the dentin tubules would become hyperconductive. Thus, their increased dentin sensitivity was due, in part, to hyperconductive dentin (Brännström 1962).
2.6 Dynamics of Smear Layer Loss and Tubule Occlusion
Many dentists ask “how long do smear layers last on planed root surfaces?” Others ask how long it takes acid-etched dentinal tubules to close by remineralization. The answer to both of these questions is “about 1–2 weeks.” In a now classic paper (Kerns et al. 1991), we used teeth extracted for periodontal reasons. Their cervical regions were root planed (Fig. 2.8a) and then divided into multiple slabs (2 × 3 × 1 mm): one served as a smear layer-covered control; another served as an EDTA-etched control with open tubules; another EDTA-etched specimen was treated with 3 % monopotassium-monohydrogen oxalate (pH 2.4) for 2 min to occlude the open tubules with crystals of calcium oxalate. Then the various dentin slabs were placed in denture flanges of denture patients for 1–4 weeks. At 1-week intervals, the dentin slabs were removed and processed for SEM.
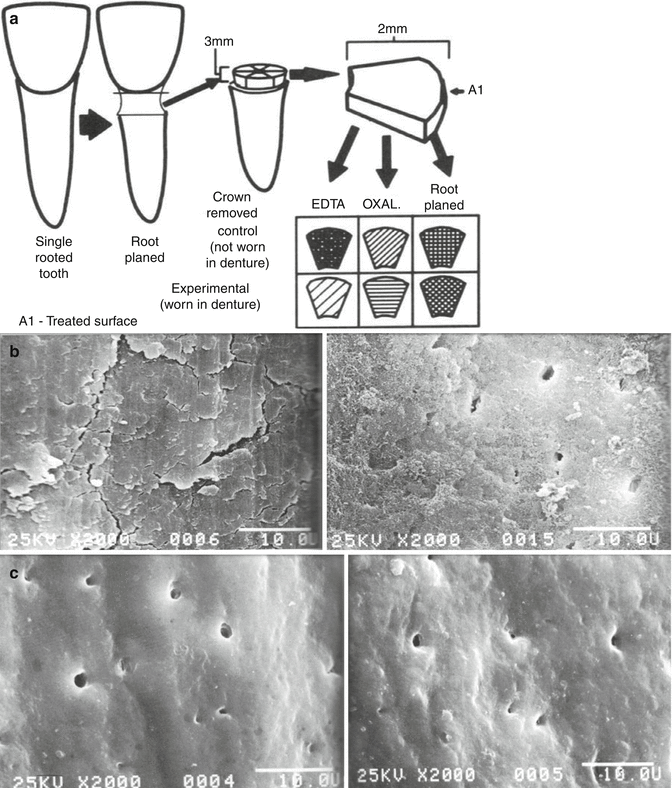
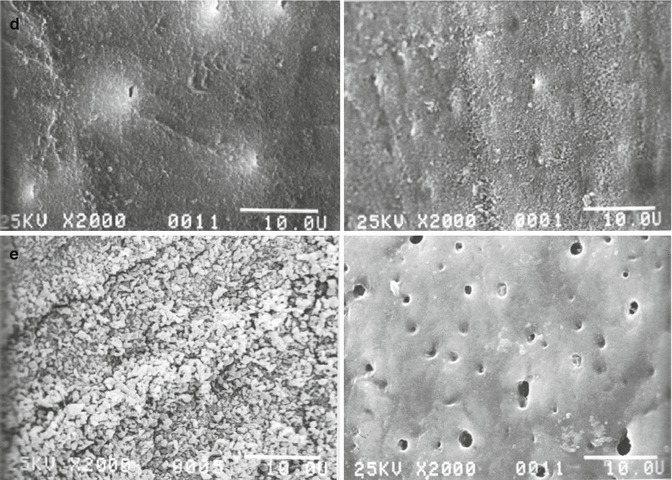


Fig. 2.8
(a–e) Kerns et al. (1991) placed dentin chips on denture flanges that were followed for up to several weeks. (b) Shows smear layer-covered dentin on the left. After 1 week, more than half the smear layer dissolved (right) opening many tubules. (c) If tubules were first opened with 18 % EDTA (left) and then exposed to saliva for a week, the tubules began to close by deposition of salivary mineral (right), which became more mineralized by 2 weeks more so (d, left) and more so after 4 weeks (right). (e) Dentin surface is covered with calcium oxalate crystals (left). After 1 week in the mouth, most of the calcium oxalate crystals on the dentin surface dissolved (right) (Kerns et al. (1991) with permission from the American Academy of Periodontology)
Figure 2.8b shows dentin covered with a smear layer before it was placed in a denture flange. The image on the right shows a similar sample that had been worn in the mouth for 1 week. Note that more than half the smear layer was lost and many open tubules were exposed. Figure 2.8c shows dentin treated with 18 % EDTA (pH 7) to remove the smear layer and expose open dentinal tubules. In the right panel is shown the appearance of similar dentin after 1 week where some of the tubules begin to be occluded by salivary salts. Fig. 2.8d show dentin that has remineralized for 2 weeks (left panel), while the right panel shows dentin that remineralized for 4 weeks. In Fig. 2.8e (left panel), dentin surfaces that were treated with 2.7 % acidic potassium oxalate for 1 min were covered with calcium oxalate crystals. In the right panel, similar dentin that had been in the mouth for 1 week showed loss of most of the calcium oxalate crystals and reappearance of some open tubules. However, some tubules remained occluded by calcium oxalate crystals below the surface.
Clinically, many patients do not complain of dentin hypersensitivity immediately after scaling and root planning (Fischer et al. 1991) but do so after 7–10 days. We believe that the lack of initial sensitivity is due to the smear layer created on root dentin by scaling; that then slowly dissolves over the next 7–10 days, making exposed dentinal tubules hyperconductive. If the patient remains untreated, these open sensitive dentinal tubules slowly begin to close by remineralization over the next 1–2 weeks. Thus, some patients may spontaneously heal but may be angry with their clinician. If patients who become hypersensitive in 7–10 days are then treated with 3 % potassium oxalate (BisBlock, Bisco Dental, Chicago, IL; SuperSeal, Phoenix, Fenton, Michigan; RemeSense), then those open tubules can be closed temporarily with calcium oxalate crystals. An alternative to these professionally applied topical oxalates is the new potassium oxalate-containing desensitizing Listerine mouthwash called Listerine Advanced Defence Sensitive (LADS) (Fig. 2.9). This is only available in England but should be available in the USA this summer.
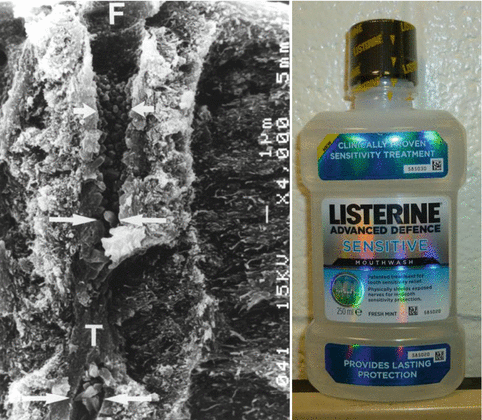

Fig. 2.9
A dentin disk with open tubules was treated with listerine advanced defence sensitive (LADS) desensitizing mouthrinse containing 1.4 wt % potassium oxalate (pH 4.2) twice a day for 60 s, for 1 week. The disk was then fractured to permit examination of intratubular contents below the surface. This scanning electron micrograph shows a funneled tubule orifice of a dentinal tubule. Acid-etching, used to open the tubule of smear layer debris removed the hypermineralized peritubular dentin matrix from the top 3−4 μm of the tubule. When treated with soluble potassium oxalate (KOx) in Listerine, the KOx diffused down the tubule until it could interact with intact peritubular dentin. The acidic (pH 4.2) mouthrinse liberated ionized calcium from the peritubular matrix, that reacted with the soluble KOx to form insoluble crystals of calcium oxalate (double white arrows). T
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


