INTRODUCTION
COMPOSITION OF AMALGAM POWDER
COMPOSITION OF AMALGAM ALLOYS
•Effects of Constituent Metals on the Properties of Amalgam
• Proportioning
CLASSIFICATION OF AMALGAM
• Generations of Dental Amalgam
• Difference between High Copper and Low Copper Alloys
SETTING REACTION OF AMALGAM
ADVANTAGES OF SILVER AMALGAM
DISADVANTAGES OF SILVER AMALGAM
INDICATIONS OF AMALGAM RESTORATION
CONTRAINDICATIONS OF AMALGAM RESTORATION
TYPES OF AMALGAM POWDER
PHYSICAL PROPERTIES OF AMALGAM
• Dimensional Change
• Strength
• Plastic Deformation (Creep)
• Corrosion
• Biocompatibility
• Thermal Conductivity
• Coefficient of Thermal Expansion
• Microleakage in Amalgam
RECENT ADVANCES IN AMALGAM
• Mercury-free Direct Filling Alloy
• Low Mercury Alloy
• Bonded Amalgam System
• Gallium in Place of Mercury in Amalgam
• Consolidated Silver Alloy
STEPS OF AMALGAM RESTORATION
• Selection of Amalgam Alloy
• Mercury Alloy Ratio
• Trituration
• Mulling
• Application of Matrix Band
• Insertion of Amalgam
• Condensation
• Burnishing
• Carving
• Finishing and Polishing
FAILURES OF AMALGAM RESTORATIONS
• Reasons for Failure of Amalgam Restorations
MERCURY HYGIENE
• History of Conflicts regarding Amalgam Use
• Forms of Mercury
• Mercury Exposure in Dental Office
• Mercury Toxicity
INTRODUCTION
The first form of silver mercury mixture was given by M Taveau in 1826 at Paris. Crawcour brothers in US introduced dental amalgam to the dentistry in 1833. Since 1850, dental amalgam has been used more than any other restorative material. The dental amalgam alloys consist of silver and other alloys like tin, copper and smaller amounts of zinc which are mixed with mercury.
Historical development of amalgam
Alloy: Alloy is a union of two or more metals
Amalgam: Amalgam is an alloy in which mercury occurs as a main constituent.
Dental amalgam: The dental amalgam is an alloy of mercury with silver, tin, and varying amounts of copper, zinc and other minor constituents.
COMPOSITION OF AMALGAM POWDER
Amalgam consists of amalgam alloy and mercury. Amalgam alloy is composed of silver-tin alloy with varying amounts of copper, zinc, indium and palladium (Fig. 9.1). Dental amalgam alloys are mainly of two types-low copper and high copper alloys.
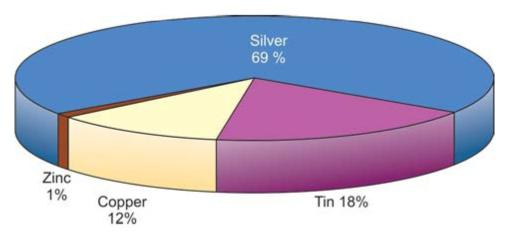
Fig. 9.1: Composition of amalgam powder
Low copper alloys contain up to 6% by weight of copper. In high copper alloys, copper content lies between 6-30%.
In general, amalgam alloy consists of silver 40% minimum, tin 32% maximum, copper 30% maximum, zinc 2% maximum, and sometimes traces of indium or palladium. In preamalgamated alloys, mercury 3% is used which react more rapidly when mixed with mercury. Mercury used for dental amalgam is purified by distillation.
Effects of Constituent Metals on the Properties of Amalgam
Silver: It has the following effects on the properties of amalgam.
• Increases strength
• Increases setting expansion
• Reduces setting time
• Resists tarnish and corrosion
• Decreases flow.
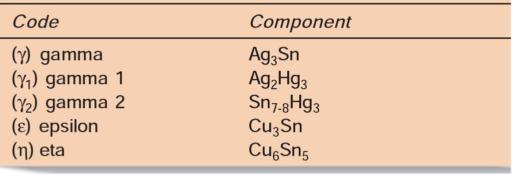
Tin: Tin helps in formation of a silver/tin compound (AgSn). This is the gamma phase which readily undergoes an amalgamation reaction with mercury. Tin causes following effects:
• Increases setting time
• Reduces strength, hardness, and setting expansion.
Copper: It has the following effects on the properties of amalgam:
• Reduces tarnish and corrosion
• Reduces creep
• Strengthening effect on the set amalgam
• Helps in uniform comminution of the alloy.
Zinc: Its presence is not essential. It may vary from 0 to 2% by weight. It has the following effects on the properties of amalgam:
• Scavanges the available oxygen to impede oxidization of Ag, Sn or Cu during alloy ingot manufacture.
• If zinc-containing alloys are contaminated with moisture, Zn gives rise to delayed or secondary expansion.
Palladium (0 to 1% by weight)
• Improves the corrosion resistance and the mechanical properties.
Indium (0 to 4% by weight)
• It decreases the evaporation of mercury and the amount of mercury required to wet the alloy particles.
Proportioning
Usually Alloy/mercury ratios range between 5: 8 and 10:8. But to achieve optimum properties of the amalgam, mercury should be less than 50%. For lathe cut alloys, it is 45%. For spherical alloys, it is 40% Hg.
COMPOSITION OF AMALGAM ALLOYS (TABLE 9.1)
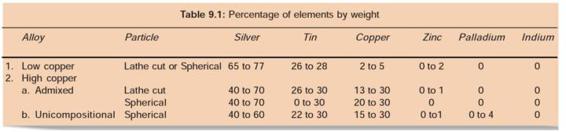
CLASSIFICATION OF AMALGAM
There are different ways of classifying amalgam alloys.
These are:
1. Based on shape ofparticles
a. Irregular: In this, shape of particles is irregular, may be in the shape of spindles or shavings.
b. Spherical: In this, shape of particle is spherical with smooth surface.
c. Spheroidal: In this, shape of particle is spheroidal with irregular surface.
2. Based on copper content
a. Low copper alloy: Copper is in range of 2-6%
b. High copper alloy: Copper is in range of 6-30%
3. Based on zinc content
a. Zinc-containing alloys: Zinc is in range of 0.01-1%
b. Zinc-free alloys: Zinc is in range of < 0.01%
4. Based on the presence of noble metals
a. Binary alloys: Contain 2 metals, i.e. silver and tin.
b. Ternary alloys: Contain 3 metals, i.e. silver, tin and copper.
c. Quaternary alloys: Contain 4 metals, i.e. silver, tin, copper and zinc.
Out of these, quaternary alloys are most acceptable.
Generations of Dental Amalgam
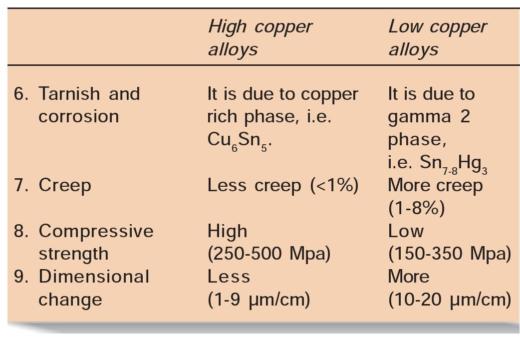
Difference between High Copper and Low Copper Alloys

SETTING REACTION OF AMALGAM
For Lathe Cut Low Copper Alloys
On mixing amalgam alloy with mercury, the alloy particles get dissolved in the mercury. In the initial reaction, mercury reacts with tin and silver without involving copper and zinc. Mercury reacts with alloy particles to form two products, i.e. the silver mercury phase and tin mercury phase. After this reaction, the unreacted particles are embedded in the matrix of reaction products with mercury. The reaction is as follows:
In lathe-cut low copper amalgams both yl and y2 form a continuous network. Since y2 phase is least corrosionresistant phase, its distribution in reaction product is important.
For Admixed High Copper Alloys
For high copper alloys, the reaction is different. It occurs in two phases. The initial reaction is similar to that of low copper alloys, i.e.
The second phase of reaction involves the silver copper phase (Ag-Cu).
It reacts with y (Ag3Sn) and mercury to form Ag2Hg3, Sn7_8Hg and Cu6Sn5 phase. The mercury released from Sn7_8Hg (y2 phase) reacts with silver to form Ag2Hg5 (Yi) phase.
This reaction goes on. After one week, the y2 phase reacts completely with eutectic and replace all the y2 phase by y and yl phase.
For Unicompositional Silver Alloy
The difference in admix type and the unicompositional alloys is that in latter the eutectic phase, i.e. Ag-Cu phase is absent. In these only silver reacts with mercury and the tin remains bound to copper.
The final phase formed is Cu6Sn5 (y) phase and there is no Ag2Hg3 (y2) phase.
ADVANTAGES OF SILVER AMALGAM
• Ease of manipulation
• Satisfactory marginal adaptation
• Wider range of application
• Physical characteristics of amalgam are comparable to enamel and dentin
• Less technique sensitive
• Self-sealing
• Biocompatible
• Good wear resistance
• Low cost
• Can be completed in one dental visit
• Bonded amalgam restorations can also bond to tooth structure.
DISADVANTAGES OF SILVER AMALGAM
• Less aesthetic
• Extensive preparation to hold an amalgam filling.
• Amalgam fillings can corrode or tarnish over time, causing discoloration.
• Does not bond to tooth.
• Being metallic restoration it is noninsulating
• Marginal degradation is seen in low copper alloys.
• Amalgam is not strong enough to reinforce the weakened tooth structure
• Poor tensile strength making it a brittle material
• Results in galvanic current in association with gold restoration or even in same restoration with nonuniform condensation.
• Oral lichen planus is also seen with amalgam restoration.
INDICATIONS OF AMALGAM RESTORATION
1. Moderate to large class I preparation.
2. Class II preparations in which there is:
• Heavy occlusion
• Extension on the root surface
• Problem of isolation.
It is indicated in heavy occlusion because amalgam has greater wear resistance than composites. Minor contamination during the amalgam placement has less adverse effects as compared to composite restorations.
3. Class V preparations in which:
• Aesthetic is not a problem
0 Preparation entirely on root surface
0 Isolation is difficult.
4. Class VI preparations.
5. Class III preparations (sometimes) where isolation is difficult.
6. Used as a foundation in cases of grossly decayed teeth while planning for cast restoration.
7. Used as a postendodontic restoration.
8. Teeth having nondefinitive pulpal prognosis-used as type of interim restoration before assessment of pulpal status of the tooth.
9. Tooth having fractured cusp can be restored with the help of amalgam using pin and slot.
CONTRAINDICATIONS OF AMALGAM RESTORATION
1. Esthetics: Use of amalgam is avoided in aesthetic areas of oral cavity. So, preparations class III, IV, V usually are not indicated except in certain cases.
2. Small to moderate class I and class II preparations should be restored with composite rather than amalgam as former results in more conservative tooth preparation.
Indications
• Moderate to large class I and II preparations
• Class V preparations (some cases)
• Class VI preparation
• Class III preparation (some cases)
• Used as foundation
• Used as postendodontic filling
• Tooth having nondefinitive pulpal prognosis
• Tooth having fractured cusp
Contraindications
• When esthetics is the prime concern
• Small to moderate class I and class II preparations
Phases of silver amalgam
TYPES OF AMALGAM POWDER
Lathe cut is made by cutting fillings of alloy from a prehomogenized ingot which was heat treated at 420°C for many hours. The fillings are then reheated at 100°C for 1 hour for aging of the alloy.
Spherical (spheroidal) alloy is formed when molten alloy is sprayed into a column filled with inert gas, this molten metal solidifies as fine droplets of alloy.
Admixed alloy is that when different size or shape of amalgam powder is mixed together to increase filling efficiency.
Single composition is that alloy in which every particle of alloy is having same shape, size and composition.
Dispersion modified, high copper alloy is that in which high copper alloy is mixed with conventional alloy.
PHYSICAL PROPERTIES OF AMALGAM
Dimensional Change
Small amount of contraction occurs in the first half an hour after trituration because mercury diffuses into the silver and tin and the mix dissolves in the mercury. After this, expansion occurs because of crystallization of the new phases. According to ADA specification no. 1 dimensional change should be limited to 20 microns/cm measured between 5 minutes and 24 hours after trituration.
Factors affecting Dimensional Changes of Amalgam
• Type of alloy being used, for example, single composition spherical alloys contract more than single composition lathe-cut or admixed alloys.
• Condensation technique, i.e. more mercury removed from the alloy, the more it will contract.
• Trituration time, overtrituration causes contraction.
• Presence of zinc can result in delayed expansion after 3-5 days of restoration, if during condensation or trituration, zinc-containing amalgam comes in contact with moisture or saliva. This occurs due to formation of zinc oxide and hydrogen gas when zinc react with water. This expansion can result in extrusion of restoration beyond preparation margins and pulpal pain.
Strength
• Strength of amalgam develops slowly. It takes 24 hours to reach maximum. In first hour, only 40 to 60% of its maximum compressive strength is achieved.
• According to ADA specification no. 1, amalgam should have minimum 1 hour compressive strength of 11,600 psi (80 MPa).
• Amalgam has much higher compressive strength than tensile or shear strength making it brittle material.
• Compressive strength of amalgam is seven times more than that of its tensile strength. Being a brittle material, it is weak in thin sections, thus unsupported edges of restoration fracture frequently. To avoid this, a 90° butt joint angle of amalgam is required at the margins.
• Spherical alloys are harder and stronger when compared to lathe-cut alloys because they require less mercury during trituration, thus less of the weak matrix portion forms.
Plastic Deformation (Creep)
• Creep is the time-dependent response of an already set material to stress. This response is in form of plastic deformation. It can be of two types depending on the stresses involved, viz. static and dynamic.
• By ADA specification no. 1, creep is limited to 3% in a set amalgam.
• Creep/flow usually occurs near the melting temperature of the material. In amalgam, creep happens because gamma 1 is a fine grained structure, also these particles “slide” across each other resulting in slipping of grain boundaries.
• Creep is unfavorable to amalgam because it causes amalgam. To flow out over margins where the thin amalgam fractures. This results in marginal deterioration.
• Factors affecting creep:
– Low copper alloys have higher creep than high copper alloys because in latter copper binds tin and forms eta phase, and this prevents formation of gamma 2 phase. Crystals of eta phase interlock and check slippage at gamma 1 grain boundaries, resulting in less creep.
– Higher the residual mercury levels, more is the creep.
– Increased condensation pressure reduces creep because it reduces residual mercury level.
– Marginal areas show more creep because they have higher levels of residual mercury.
– A delay between trituration and condensation increases creep.
Corrosion
• Amalgam restoration shows corrosion and tarnish over a period of time. Though corrosion causes decrease in strength of a restoration by 50% in five years, the advantageous fact of corrosion is that the byproducts that form, act to seal the preparation margin, responsible for self-sealing feature of amalgam (Fig. 9.2).
• In low copper amalgams, the most corrosion-prone phase is gamma 2 (Sn7_8Hg3) phase. In these alloys, corrosion products are tin oxides and tin chlorides. Here the corrosion proceeds from outer surface to interior of restoration making it porous and spongy.
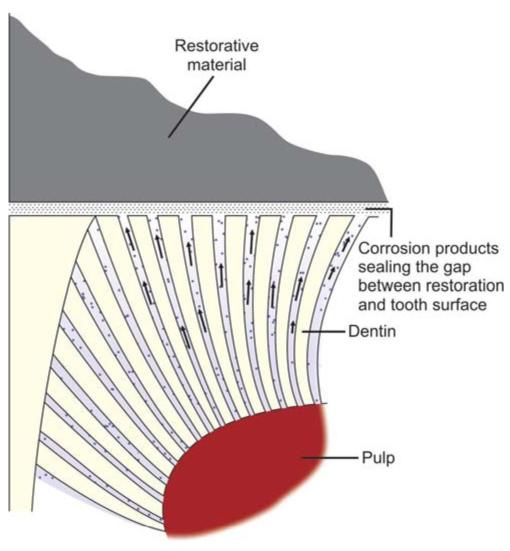
Fig. 9.2: Sealing of restorative margins because of corrosion products of amalgam
• In high copper amalgams, corrosion products are similar to that of low copper alloys, in addition there is formation of copper chloride which corrode slower than low copper amalgams. High copper alloys corrode slower because they contain little or none of gamma 2 phase. Also the corrosion is not of penetrating type as in low copper alloys. In high copper alloys, the most corrosionprone phase is the eta phase.
Biocompatibility
Though there has been great debate related to mercury toxicity, yet if careful handling of mercury is taken, amalgam proves to be a biocompatible material.
Thermal Conductivity
Because of good thermal conductivity, amalgam can transmit temperature changes readily to the pulp. Hence, its closeness to pulp should be avoided without adequate pulp protection.
Coefficient of Thermal Expansion
It is three times more in amalgam than that of dentin. This large difference is responsible for microleakage.
Microleakage in Amalgam
Microleakage occurs when there is 2 to 20 micron wide gap between the amalgam and tooth structure.
Following factors are responsible for microleakage in amalgam:
• Poor condensation techniques that cause marginal voids.
• Lack of corrosion byproducts which are necessary for sealing of margins.
• High coefficient of thermal expansion of amalgam.
• Use of single composition spherical alloys which show more leakage than lathe-cut or admixed alloys.
Microleakage can lead to (Figs 9.3A and B):
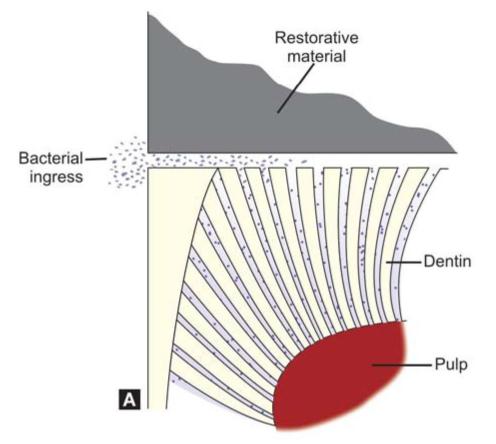
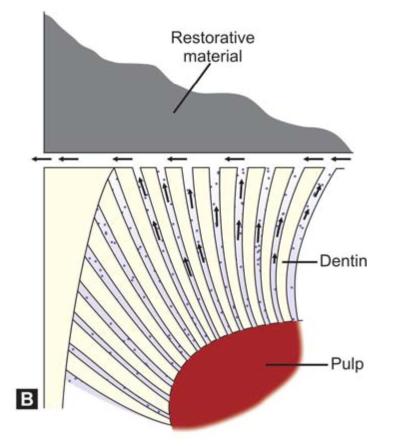
Figs 9.3A and B: (A) Bacteria can pass into the marginal gap causing pulpal inflammation, (B) The dentinal fluid can come out of the marginal gap causing pain and sensitivity
• Pulpal inflammation
• Tooth discoloration
• Postoperative sensitivity
0 Restoration failure
RECENT ADVANCES IN AMALGAM
Mercury-free Direct Filling Alloy
The American Dental Association, in combination with National Institute on Standard and Technology (ADA-NIST) has patented a mercury-free direct filling alloy which is based on Ag-coated Ag-Sn particles that can be self-welded by compaction to create a restoration. To keep the surface of alloy particles clean, a fluoroboric acid solution is used. These alloys can be condensed in the same manner as direct filling gold restoration.
Low Mercury Alloy
In this approach, alloy particles are carefully selected so that they can pack together well. Then it is possible to reduce the mercury content for mixing to the 15-25% range. However, the clinical properties of these alloys are not yet known.
Bonded Amalgam System
One of the major disadvantages of the amalgam is that it does not adhere to the preparation walls. To conquer this problem, bonding systems to bond the amalgam to tooth structure have been developed. In the bonded amalgam technique, a dentin bonding system is used along with a viscous resin liner which physically mixes with the amalgam and forms a micromechanical union to increase amalgam’s retention to tooth structure.
Since amalgam is hydrophobic, and tooth is hydrophilic, therefore, to achieve optimal wetting, bonding systems must have dual properties. For this monomer molecule having hydrophilic and hydrophobic ends of 4-methyloxy ethyl trimellitic anhydride (4-META) based systems are used.
Indications of Bonding
• When remaining tooth structure, after tooth preparation is weak.
• In extensively carious posterior teeth where it acts as cost effective alternate for cast metal and metal ceramic restorations.
• In cases with deep bite where short clinical crown is present, here pin-retained restorations are not possible. In these cases, bonding provides auxiliary retention.
• As core for foundation of cast crown restoration.
Advantages of Using Bonded Amalgam System
• Adequate dentin sealing
0 Increased resistance form
• Increased retention
• Conservative tooth preparation
• Improved marginal seal
• Elimination of use of retention pins and other modes of retention.
• Reduction in microleakage, secondary caries and postoperative sensitivity.
• Cost effective for extensively carious tooth
• Can be done in single appointment.
Limitations of Amalgam Bonding
• Reduction in bond strength over years because of repeated thermocycling in the oral cavity.
• Technique sensitive system, amalgam must be condensed over wet adhesive resin.
• Expensive than nonbonded amalgam restoration.
Technique of Bonded Amalgam
Bonding agents used in amalgam bonding system: Although many products are available for adhesion to enamel and dentin, most of these are meant for use with resin composites. Some of them have metal bonding capabilities thus can be used for amalgam bonding. These are Amalgambond Plus with HPA (High Performance Additive) powder (Parkell), Optibond 2 (Kerr), All Bond 2 (Bisco), Panavia EX and Panavia 21 (Kuraray).
Bonding interface (Figs 9.4 and 9.5): The bonding interface constitutes tooth, amalgam and adhesive resin present in between them.
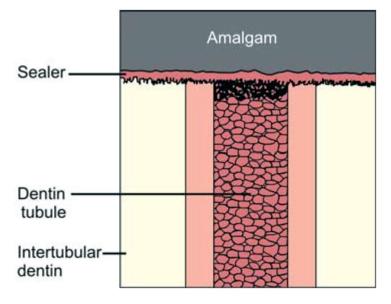
Fig. 9.4: Bonding interface of amalgam and tooth: Conventional amalgam restoration
The bonding interface may consist of the following:
1. Tag formation
2. Precipitates on pretreated dentin surfaces to which adhesive resin binds mechanically or chemically.
3. Formation of hybrid layer of reinforced dentin.
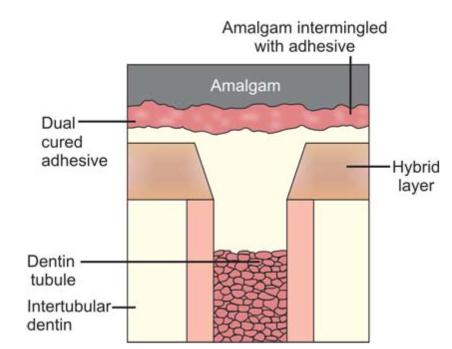
Fig. 9.5: Bonding interface of amalgam and tooth in bonded amalgam restoration
4. Chemical binding to the inorganic and organic components of dentin and enamel.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


