(1)
State Key Laboratory of Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, 610041, Sichuan, China
Keywords
Ectodermal layerEndodermal layerMesodermal layerNeural crest cellsEctomesenchymePrimitive stomatodeumEnamelAmelogenesisHistogenesisMorphogenesisCytodifferentiationDentin–pulp complexDentinPredentinPulpOdontoblastHertwig’s epithelial root sheath (HERS)Epithelial–mesenchymal transition (EMT)Signaling pathwayTooth eruption
1.1 Embryology of the Craniofacial Tissues
The development of craniofacial tissues is part of human prenatal development. Generally, human prenatal development goes through three stages: the proliferative 2-week period, when cell division is prevalent; the embryonic period, which extends from the second to the eighth weeks; and the fetal period, from eighth week to birth [1]. With normal accomplishment of the development, human body forms stepwise.
1.1.1 Origin of Human Tissue
The origin of tissue begins with fertilization, which is the fusion of spermatozoa and ova to form a zygote. Then the zygote moves to the uterine cavity where it will implant into the wall of the uterus and, meanwhile, undergoes a series of rapid divisions that lead to the formation of a fluid filled hollow ball, termed blastocyst, and small inner cell mass. When this blastocyst attaches to the sticky wall of the body of the uterus, uterine endometrium is digested, allowing blastocyst embedded in its surface and then deeper penetration. Implantation takes place.
On either side of the inner cell mass, two small cavities are formed. A small disk (the embryonic disk) develops in the center, where they reach each other. The embryonic disk becomes the embryo, composed of two layers of cells. One layer is lined with ectodermal cells, which will form the outer body covering (epithelium), called ectodermal layer. The cells on the ventral aspect are endodermal cells, forming the endodermal layer. This configuration is completed in the first 2 weeks, which is termed “proliferative period” [1].
During the third week, two-layered embryonic disk is converted to a three-layered disk. Cells that develop between the ectodermal and endodermal layers become the mesodermal layer. Next, major tissues and organs, including oral maxillofacial tissue such as tooth and facial bones, differentiate from these three layers [2]. Key events are the development of the nervous system, differentiation of neural crest tissue from the ectoderm, and folding of the embryo.
1.1.2 The Neural Crest
The nervous system begins with a specification of the neural plate, which develops as a thickening within the anterior ectodermal layer. Meanwhile, the neural plate develops raised folds at its margins. These folds in turn encompass and fuse so that neural tube forms and separates from the ectoderm.
Upon closure of the neural tube, a unique population of cells known as neural crest cells separate from the lateral aspect of the neural plate. These cells have the capability of migration and differentiation. This is especially obvious in the head and neck region, and neural crest cells have an important role in the head development. They contribute to most of the embryonic connective tissue of facial region, which includes dental tissues such as the pulp, dentin, and cementum. Consequently, embryonic connective tissue in the head is termed as ectomesenchyme, reflecting its origin from the ectoderm, whereas connective tissue elsewhere is derived from the mesoderm and is known as mesenchyme. Although the neural crest tissues arise from neural ectoderm, they exhibit properties of mesenchyme [2, 3].
1.1.3 Head Formation
The head fold of the three-layered embryo is crucial and produces the primitive stomatodeum or oral cavity. When the stomatodeum first forms, it is surrounded by frontal prominence rostrally and by the cardiac bulge caudally. And it is separated from the foregut by buccopharyngeal membrane, a bilaminar structure consisting of ectoderm and endoderm, which breaks down soon so that the stomatodeum communicates with the foregut. Laterally the somatodeum becomes delimited by the first pair of pharyngeal arches [1, 2].
1.2 Enamel Development
Fully mature enamel comprises 80–90 % (v/v) carbonated calcium hydroxyapatite crystals, which is in contrast to bone and dentin, both with 10 % and 13 % (v/v) carbonated calcium hydroxyapatite crystals, respectively. The mechanisms of crystal initiation, crystal growth, as well as morphology are related to amelogenesis. In developing teeth, sequential and reciprocal interactions occur between the epithelium and the underlying mesenchyme, which derive from the cranial neural crest. Enamel formation associates with the differentiation of the tooth-specific cell types, the epithelial ameloblasts. This chapter will provide a brief overview in different aspects of tooth enamel development with a particular emphasis on the current knowledge of enamel morphogenesis, histogenesis, and cytodifferentiation.
1.2.1 Histogenesis and Morphogenesis
The consecutive phases during tooth morphologic changes, including lamina, bud, cap, and bell stages, are characterized by epithelial histogenesis. The segmentation of the dental epithelium occurs in the early tooth initiation, which indicates that a local epithelial thickening corresponds to the dental lamina. Experiment approaches suggested that Wnt/Shh interactions may regulate the delimitation between the dental epithelium and the oral ectoderm. Nevertheless, the molecules intervene in regulating epithelial cell apoptosis, and survival or compartmentalization of different elements is still poorly understood (Fig. 1.1).
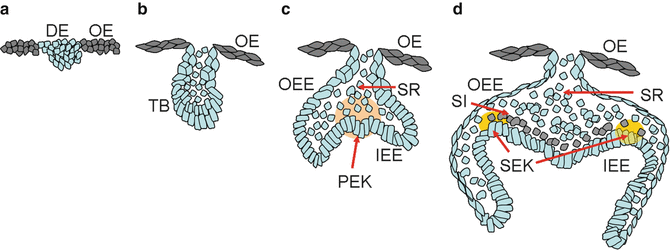

Fig. 1.1
(a–d) Morphogenesis of the tooth development (from bud to bell stage)
1.2.1.1 Bud Stage
From the bud stage, the thickened presumptive dental epithelium, which forms the basal epithelium, can be distinguished from the round internal cells. Depending on the position, epithelial cells are evident in changes of the expression of different molecules, including γ-catenin, desmoglein, and P- and E-cadherins.
1.2.1.2 Cap Stage
During the cap stage, the dental epithelium becomes the enamel organ which consists of four different cell types: inner and outer dental epithelia, the stellate reticulum, as well as transiently the primary enamel knot. At this time, the inner enamel epithelium becomes discernible from the outer enamel epithelium. The histogenesis of the inner dental epithelium is coordinated by a change in the composition of the basement membrane.
The enamel knot is a dynamic transient structure and appears at the onset of mammalian tooth shape development, which is in contact with several cells, including peripheral cells, internal round cells, and basement membrane cells. Studies indicated that the primary enamel knot represents a signaling center in formatting cusps, which may lead to unequal growth of the enamel epithelium and induce the formation of secondary enamel knots.
It has been suggested that the structure and organization of primary enamel knot rapidly change during the time. At the beginning of the cap stage, it appears as a long cylindrical shape and the shape will extend along the mesial–distal axis of the first lower molar. Soon after, some of internal cells begin apoptosis. While the first lower molar grows during cap formation, the primary enamel knot starts to extend in anterior and posterior directions. It is suggested that in the primary enamel knot, most cells do not divide and its proliferation needs the recruitment of cells within the enamel organ. However, the underlying mechanism is still not known due to differences in mouse strains or measuring stages of embryos.
1.2.1.3 Bell Stage
At the bell stage, the enamel organ delimitates the dental papilla and starts to form cusps. At this time, the secondary enamel knots form, which only precede cusp formation by a few hours. The secondary enamel knots are taken place at the tips of the forming cusps at the bell stage. The relationship between primary and secondary enamel knots has been suggested in several models. The gene expression patterns elucidated the primary enamel knot induces the secondary enamel knots by a reaction–diffusion-related mechanism.
During the bell stage of tooth development, the shape of the crown is determined. The growth of crown results from cell division and reorganization of inner dental epithelium. Furthermore, the programmed cell death is also accompanied by the regulation of cell number in the inner dental epithelium, suggesting its role in determining the final number of functional ameloblast cells.
1.2.2 Cytodifferentiation
From the lamina stage to the bell stage, changes in reorganization of the epithelium compartment can be distinguished. They not only regulate histogenesis but also determine the final number and specific positioning of functional ameloblasts.
Amelogenesis, or enamel formation, consists of two main steps. The first step creates partially mineralized enamel (about 30 %). The second step involves extreme influx of additional mineral while removing organic material and water to form more than 96 % mineral contents. The differentiation of epithelial cells into functional ameloblast cells includes several morphologic changes that occur in time and involve growth, elongation of the cytoplasm, polarization, and secretion of matrix protein. These epithelial cells exhibit a unique character of progressively changed phenotype. Amelogenesis has been described in as many as six stages but generally is divided into three functional phases, known as presecretory, secretory, and maturation stages (Fig. 1.2).
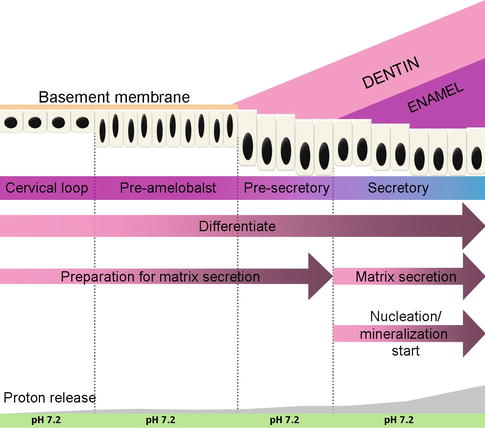

Fig. 1.2
Ameloblast differentiation
1.2.2.1 Presecretory Stage
During the presecretory phase, the cells of the inner enamel epithelium start to differentiate into ameloblast cells. At the morphogenetic phase, inner enamel epithelium cells are cuboidal or low columnar, with large centrally located nuclei and poorly formed organelles in the proximal portion of the cells. During differentiation phase, once stimulated, these cells elongate, their nuclei shift distally toward the stratum intermedium, and the Golgi elements increase and migrate distally. Moreover, the cytoplasm becomes filled with organelles which are needed for the synthesis and secretion of enamel proteins. At this time, a second junctional complex forms at the distal extremity of the cell, compartmentalizing the ameloblast cells into a body and a distal extension called Tomes’ process, against which enamel develops.
Although the pre-ameloblasts have been regarded as nonsecreting cells, more and more researches demonstrate that the enamel protein secretion starts much earlier, even before the separation between pre-ameloblasts and pre-odontoblasts. Ameloblast cells are aligned closely with each other due to the tight junctional complex or attachment specializations [2]. These junctional complexes greatly involved in amelogenesis determine what may pass between cells to enter or leave the enamel at different times.
1.2.2.2 Secretory Stage
The newly formed ameloblasts near the dental papilla are flat and the matrix secreted is called rodless enamel matrix. During the secretory stage, the ameloblasts exhibit a tall columnar and polarized morphology and secrete an extracellular protein-rich matrix. The fine structure of secretory stage ameloblasts indicated their strong synthetic and secretory activity. The Golgi complex is intense and forms a cylindrical organelle surrounded by many cisternae of rough endoplasmic reticulum. Ameloblast secretion is constitutive, which means the secretion is successively, and the secretory granules are not stored for prolonged periods of time.
When enamel formation begins, Tomes’ process comprises only a proximal portion. The secretory granules are released along the surface of the process against the newly formed mantle dentin to create an initial layer of the enamel without enamel rods. The very first hydroxyapatite crystals formed interdigitate with the dentin crystals. After forming the initial enamel layer, ameloblast cells migrate from the dentin surface and form the distal portion of Tomes’ process as an outgrowth of the proximal portion. The distal portion extends into and interdigitates beyond the initial layer of enamel, while the proximal portion penetrates from the distal junctional complex to the enamel layer surface [2].
It is believed that the distal portion of Tomes’ process progressively lengthens as the enamel layer thickens and gradually turns to be thinner as the rod developing in diameter presses it against the wall of the interrod cavity. Eventually, the process is squeezed out of existent, leaving a narrow area which is filled with organic materials between the enamel rod and interrod enamel. When the outer layer of enamel is being formed, the distal portion of Tomes’ process is altered and orientation also changed, leading to slightly difference of enamel rods in the outer third of layer with a more rectilinear trajectory. Finally, the ameloblasts become the same overall appearance as when initial enamel was forming. Without the distal portion of Tomes’ process, the final enamel has no rods. Notably, the initial, interrod, and final enamels are developed by the same secretory surface and, indeed, form a continuum [2].
1.2.2.3 Maturation Stage
During the maturation stage, the ameloblasts aim at resorbing much of the water and organic matrix from the enamel in order to allow enough space for the growing enamel crystals [2]. This change results from the thickness and width growth of preexisting crystals seeded during amelogenesis formative stage, not due to additional crystal accumulation.
It is believed that the stratum intermedium cells are also related to secretory and absorptive functions of amelogenesis and desmosomes facilitate their close contact with ameloblast cells. The stratum intermedium cells appear less active as enamel maturation is near completion [4].
After immature enamel has fully formed, ameloblast cells undergo several morphologic changes in preparing maturing the enamel. At this time, a short transitional stage appears, during which ameloblasts become shorter and their volume and organelle content decrease. At the maturation stage, some ameloblast cells undergo programmed apoptosis; roughly about half of the ameloblasts is reduced during amelogenesis.
In summary, ameloblasts arise from the inner enamel epithelial cells and experience multiple morphologic and functional changes. Following the deposition of a layer of enamel, ameloblasts deposit enamel in the form of rods or prisms that become highly mineralized. The arrangement of ameloblasts with their Tomes’ process plays a critical role in the formation of enamel rods. The process of amelogenesis is a series of successive phases of proliferation, differentiation, secretion, and maturation, eventually forming the enamel.
1.2.3 Microstructure of the Enamel
The enamel is a composite structure consisting of mineral and organic phases. At the nanometer scale, like most other naturally mineralized tissues, dental enamel has hierarchical structures and surface features [5, 6]. On the microscale, the enamel consists of highly organized architectural units known as enamel prisms. On the nanoscale, the enamel consists of highly crystalline nanorod-like calcium hydroxyl apatite crystallites that are arranged roughly parallel to each other [7] (Fig. 1.3).
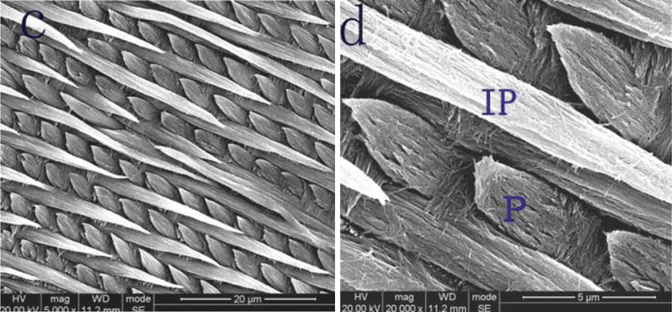

Fig. 1.3
Enamel structures
1.2.3.1 Enamel Rod
Using the scanning electron microscope and following a short etching part, enamel rods can be observed in ground or fractured teeth. The enamel rod represents the mineralized progress of ameloblasts and Tomes’ process. Enamel rods cross one another and follow an undulating course as they progress from the DEJ toward the enamel surface. When the arcades connect to each other, enamel rods have the features of keyholes or paddles, with the convex surface of the arcades oriented in an incisal or cuspal order. The enamel rods run nearly perpendicular to all parts of tooth surface, stopping at the final layer of aprismatic enamel [4].
1.2.3.2 Enamel Spindle
Enamel spindles are generated during the differentiation stage of amelogenesis. When the initial enamel is formed, the enamel spindles become terminal extensions of the primary dentinal tubule into the enamel matrix. Spindles exhibit bulbous structures at DEJ region in mature tooth enamel.
1.2.3.3 Enamel Lamellae and Cracks
It is believed that enamel lamellae are the result of local failure of the maturation process. Enamel lamellae include thin sheets of organic materials that extend throughout the enamel mineralization and exhibit vertical orientation from incisal or cuspal regions to cervical area. Cracks share some similar characters with lamellae in ground section and usually appear as artifacts during teeth processing.
1.2.3.4 Enamel Tufts
Enamel tufts originate from the DEJ and extend 1/3 to 1/2 of the thickness of the enamel matrix. They are formed during Tomes’ process development and also during the elaboration of the initial enamel of the enamel rod. They represent protein-rich regions that failed to mature in the enamel matrix.
1.2.3.5 Interpit Continuum
The secretory product is released from the ameloblast cells at two preferred sites. The comparatively superficial site forms the majority parts of all developing enamel surfaces. This site is interameloblastic and the product determines pits, naming interpit for this stage. In many circumstances, the interpit phase is continuous throughout vast parts of the tissue [8]. The second location at which the enamel matrix is released is from the secretory pole of Tomes’ process proper, which aims at filling in the pit. At these sites, crystals may have orientations that merge with those from the interpit stage, building open-sided prism boundaries.
1.2.3.6 Functional Aspects of Enamel Structure
The particular organization of the enamel serves as enhancing hardness and wear resistance. The parallel formation of crystals perpendicular to the surface of the teeth brings about the best way for dense packing of the crystals as well as obviates the need to nucleate new crystals during enamel maturation. It has a microporous structure, which allows extra mineral flow in the crystals for further growth while degrading matrix components to be removed. Although single crystal is too flexible, the perpendicular positions enable the growth of long whisker-like crystals, allowing the crystals form into larger domains to be stronger and stiffer.
Enamel crystals are the largest crystals found in the body. The primary structural unit of enamel is the enamel rod, which is formed by the secretory activity of ameloblasts. The orientation of crystals and the distribution of organic matrix are involved in maintaining structural properties of enamel.
1.2.4 Enamel Matrix Proteins
Enamel formation requires a remarkable orchestration of diverse and essential enamel-secreted proteins, including amelogenin, ameloblastin, enamelin, amelotin, tuftelin, dentin sialoprotein, and apin. Studies provide functional data showing that the disruption of synthesizing, secreting, and processing these genes can cause different subtypes of amelogenesis imperfecta (AI), indicating the indispensable role for enamel composition and maturation [9].
1.2.4.1 Enamelin
A number of studies have suggested that the first protein to be secreted by ameloblasts at the dentin–enamel junction (DEJ) region is enamelin [10, 11]. Enamelin is a novel acidic enamel protein that has been postulated to play an essential role in enamel mineralization. By high-resolution protein-A gold immunocytochemistry, the acidic feature of enamelin proteins has been reported to be in line with its capability to bind to enamel mineral crystallite surfaces [12]. Enamelin is rich in aspartic acid and could be arranged in β-sheet conformation that results in nucleation of the mineral component.
The enamelin proteins initially secreted at the very early phase of enamel formation are strictly expressed by ameloblasts and persist throughout enamel developing and maturing stages [10]. The mutations of enamelin such as enamelin-null phenotype are associated with aberrations of enamel, causing AIH2. Several studies have described the mutations of enamelin gene causing an autosomal-dominant AI phenotype [13]. In Enam−/− mice, the enamel layer is completely absent. The crust over the dentin is thin, irregular, and easily abraded [10]. These analyses indicate that enamelin is essential for enamel matrix organization and mineralization.
1.2.4.2 Amelogenin
The amelogenin proteins of developing dental enamel are tissue-specific components, rich in leucine, histidine, proline, and glutamyl residues. Among all the ameloblast-specific proteins, amelogenin is the most abundant extracellular protein. The initial enamel layer is dominated by amelogenin protein secretion. In human, the amelogenin gene has been shown to be located on both X and Y chromosomes [14]. Human amelogenin genes have 7 exons, with the principal variation of sequence homology occurring within exon 6, which codes for most amelogenin core and the C-terminus [15].
It has been shown that the amelogenin nanospheres, the supramolecular assembly of amelogenin, such as elastin, appear as a functional structural protein during enamel formation [16]. Two human pedigrees with an X-linked AI (AIH1) phenotype both share the same mutation in the amino-terminal, tyrosine-rich amelogenin peptide (TRAP) segment [17, 18]. The recombinant proteins of those two AIH1 point mutations have been compared with wild-type amelogenin, exhibiting altered nanosphere dimensions and amelogenin assembly kinetics [19, 20]. During in vivo enamel formation, the amelogenin nanosphere also can be observed adjacent to HAP crystallites [21].
It has been found that human-inherited enamel defect AI often associates with alterations in amelogenin X chromosome gene [22]. The mutations in amelogenin are known to hypoplastic or hypomineralized enamel [22, 23]. Amelogenin knockout mice also display abnormal teeth with chalky-white discoloration, broken tips of incisors and molars, as well as disorganized hypoplastic enamel, indicating amelogenin proteins play a major role in the regulation of enamel thickness and organization of crystal pattern [24] (Fig. 1.4).
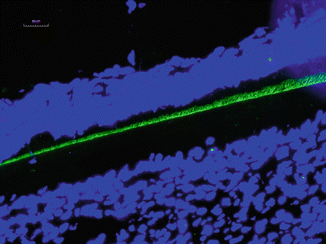

Fig. 1.4
Immunostaining of amelogenin
1.2.4.3 Ameloblastin
Ameloblastin, a cell adhesion molecule, is one of the unique tooth-specific proteins, expressed by secretory ameloblasts, yet the expression decreases during enamel maturation [25]. Shortly after dental epithelium initial differentiation, the cells are detached from the underlying matrix, resume proliferation, and lose polarity, reversing to undifferentiated one, indicating that ameloblastin maintains the differentiation state of ameloblasts at the secretory stage, by binding to ameloblasts and by inhibiting their proliferation [26].
At secretory amelogenesis, ameloblastin distribution following the ameloblast cell outline appears to be a ‘fishnet’-like partitioning [27]. The ameloblastin null mice reveal severe enamel hypoplasia, and overexpression of ameloblastin in the enamel organ influences enamel crystallite habit and enamel rod morphology, resulting in a phenotype characteristic of AI. Undoubtedly, these data all suggest that in the enamel matrix, either gain of function or loss of function of ameloblastin can cause enamel alterations. It has also demonstrated that ameloblastin acts as a nucleator of crystallization because it is expressed at mineralization initiation sites within the enamel (Fig. 1.5).
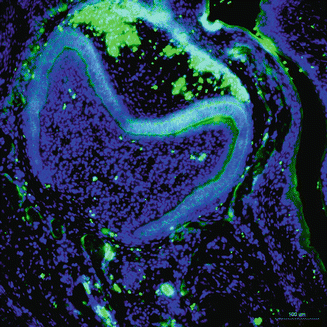

Fig. 1.5
Immunostaining of ameloblastin
1.2.4.4 Amelotin
Murine amelotin has been identified recently, which is the newest described enamel-specific protein. In developing murine incisors and molars, expression of amelotin mRNA was restricted to maturation-stage ameloblasts [28]. Both murine and human amelotin genes contain 9 exons and 8 introns and are located on chromosomes 5 and 4q13.3, respectively, which are close to the enamelin and ameloblastin genes. The expression of amelotin mRNA is essentially limited in postsecretory ameloblasts, experiencing a dramatic increase from secretory to maturation phase ameloblasts and subsequently lessening toward the zone of reduced ameloblast cells. Less information is available describing whether or not amelotin is a candidate gene for AI.
1.2.4.5 Tuftelin
Shortly after differentiation, tuftelin, an acidic protein, is synthesized and secreted. Tuftelin gene localizes to chromosome 1q21 in human. In secretory stage, the secretory pathway of amelogenin to the extracellular space is from the Golgi complex and then to Tomes’ processes [29]. However, in vivo tuftelin accumulates in cytoplasmic area other than the Golgi complex and secretes granules in both mineralizing and nonmineralizing tissues.
1.2.4.6 Proteolytic Enzymes
There are two major proteinases secreted into the enamel matrix, including matrix metalloproteinase-20 (MMP20, enamelysin) and kallikrein-4 (KLK4, enamel matrix serine proteinase-1, or serine proteinase 17).
Matrix Metalloproteinase-20
Human MMP20 gene consists of 10 exons and is part of the MMP gene clusters. The human MMP20 is located on chromosome 11q22.3, and an autosomal-recessive form of AI was recently discovered in a family that had a mutation in the intron 6 splice acceptor [13]. In porcine teeth, both ameloblast and odontoblast cells express MMP20. During the early stage of enamel formation, MMP20 activity accounts for virtually all of the known cleavage sites in amelogenin. The mutation of MMP20 exhibits hypoplastic enamel with improperly processed amelogenin and rod pattern [30]. In addition, the homozygous MMP20 mutation family reveals severely pigmented, brittle, and soft enamel, which is characterized by less radiodense.
Kallikrein-4
Human KLK4 gene is located on chromosome 19q13.41. KLK4 was first discovered in the teeth, but it also expressed in other tissues such as the prostate. In the teeth, KLK4 is secreted by different cell types, including odontoblasts and late-secretory and maturation phase ameloblasts [31]. KLK4 expression during enamel maturation correlates with the degradation of enamel proteins, thus indicating it is necessary for the enamel to achieve the high level of mineralization. KLK4 mutation was found in a family with autosomal recessive hypomaturation AI, showing yellow-brown discolored teeth. The enamel fractured from the teeth has normal thickness but with a decreased mineral content. Notably, the affected members are all females, so it is not sure whether KLK4 has an effect on the prostate. However, only the teeth were apparently altered by the homozygous KLK4 mutation [23].
1.3 Pulpodentin Complex
In a mature tooth, dentin is a unique, avascular mineralized connective tissue that forms the bulk of the tooth, and dentin encloses a richly innervated and highly vascularized soft connective tissue, the dental pulp. Dentin and pulp are derived from the dental papilla, whose cells migrate from the cranial neural crest. The tissues remain closely associated during development and throughout the life of an adult tooth and are hence most commonly referred to as the “pulpodentin complex.”
During the process of tooth development, most attentions are focused on the common themes about odontoblast differentiation that have emerged and what is known about the influence of tooth-signaling molecules and transcription factors on the development and homeostasis of the pulpodentin complex. In addition, the focus is the theories about the general principles of dentin matrix formation, particularly the synthesis and secretion of extracellular matrix molecules and their postulated roles in the biomineralization of dentin, and the theories about the development and homeostasis of differentiated and undifferentiated or stem cell populations can be translated to regenerative approaches targeted at restoring the integrity of the adult pulpodentin complex.
1.3.1 Dentin
Fully mature dentin is composed of approximately 70 % inorganic material and 10 % water by weight. The principal inorganic component consists of Ca10(PO4)6(OH)2 (hydroxyapatite). Organic matrix accounts for 20 % of dentin. 91 % of organic matrix is collagen, and most of the collagen is type I, with a minor component of type V. Noncollagenous matrix components include phosphoproteins, proteoglycans, gamma-carboxyglutamate-containing proteins (Gla-proteins), acidic glycoproteins, growth factors, and lipids. By volume, inorganic matter makes up 45 % of dentin, while organic molecules and water 33 % and 22 %, respectively. A characteristic of human dentin is the presence of tubules that occupy from 20 to 30 % of the volume of intact dentin. These tubules house the major cell processes of odontoblasts. The elasticity of dentin provides flexibility for the overlying brittle enamel.
1.3.1.1 Structure of Dentin
Dentinal Tubules
The characteristic of dentin is the presence of tubules, which host the major cell processes of odontoblasts. Tubules form around the odontoblast processes and thus transverse the entire width of the dentin from the DEJ or DCJ to the pulp. They are slightly tapered in the wider portion situated toward the pulp. This tapering is the result of the progressive formation of peritubular dentin, which leads to a continuous decrease in the diameter of the tubules toward the enamel.
In coronal dentin, the tubules have a gentle S shape as they extend from the DEJ to the pulp. The S-shaped curvature is presumably the result of the crowding of odontoblasts as they migrate toward the center of the pulp. As they approach the pulp, the tubules converge because the surface of the pulp chamber has a much smaller area than the surface of dentin along the DEJ.
The number and diameter of the tubules are different at various distances from the pulp, and the mean number and diameter of tubules decrease following the increased distance (Fig. 1.6) [32]. Investigators found the number and diameter of dentinal tubules to be similar in rats, cats, dogs, monkeys, and humans, indicating that mammalian orthodentin has evolved amazingly constantly [33].
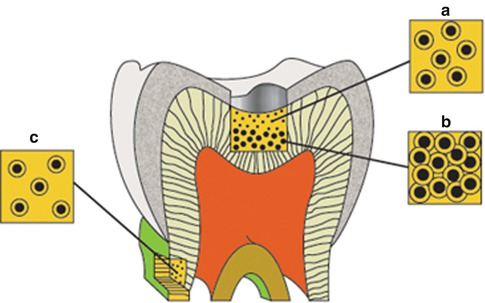

Fig. 1.6
Diagram illustrating the difference in size and number of tubules on the occlusal surface of coronal dentin (A and B) and at the cervical region of the root surface (C). This combination is responsible for the exponential increase in dentin permeability with depth (From Pashley [32], p. 106, figure 2)
Near the DEJ, the dentinal tubules ramify into one or more terminal branches; this is due to the fact that during the initial stage of dentinogenesis, the differentiating odontoblasts extended several cytoplasmic processes toward the DEJ, but, as the odontoblasts withdrew, their processes converged into one major process (Fig. 1.7).
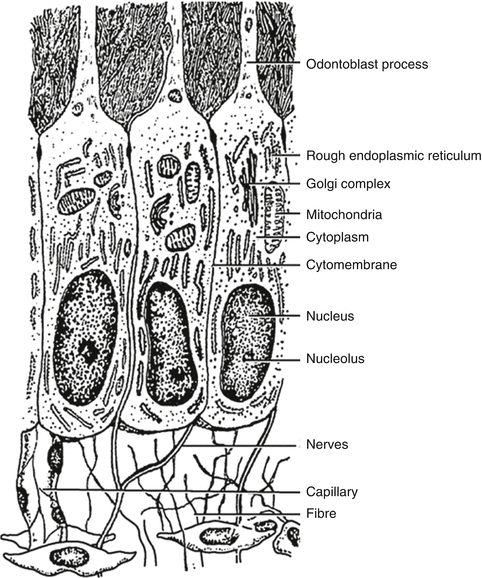

Fig. 1.7
Diagrammatic representation of the differentiated odontoblast
Peritubular Dentin
Dentin lining the tubules is termed peritubular dentin, whereas that between the tubules is known as intertubular dentin (Fig. 1.8). Presumably precursors of the dentin matrix that is deposited around each odontoblast process are synthesized by the odontoblast, transported in secretory vesicles out into the process, and released by reverse pinocytosis. With the formation of peritubular dentin, there is a corresponding reduction in the diameter of the process.
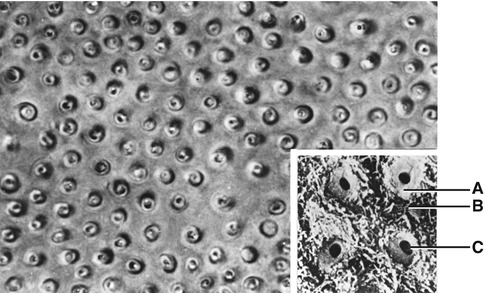

Fig. 1.8
The cross section of dentinal tubules. (A) Peritubular dentin; (B) intertubular dentin; (C) dentinal tubule
Peritubular dentin represents a specialized form of orthodentin not common to all mammals. The matrix of peritubular dentin differs from that of intertubular dentin in having relatively fewer collagen fibrils and a higher proportion of sulfated proteoglycans. Because of its lower content of collagen, peritubular dentin is more quickly dissolved in acid than is intertubular dentin.
Peritubular dentin is more highly mineralized and therefore harder than intertubular dentin. The hardness of peritubular dentin may provide added structural support for the intertubular dentin, thus strengthening the tooth. By preferentially removing peritubular dentin, acid etching agents used during dental restorative procedures enlarge the openings of the dentinal tubules, thus making the dentin more permeable.
Intertubular Dentin
Intertubular dentin is located between the rings of peritubular dentin and constitutes the bulk of circumpulpal dentin. Its organic matrix consists mainly of collagen fibrils having diameters of 500–1000 Å. These fibrils are oriented approximately at right angles to the dentinal tubules.
Interglobular Dentin
The term interglobular dentin refers to organic matrix that remains unmineralized because the mineralizing globules fail to coalesce. This occurs most often in the circumpulpal dentin just below the mantle dentin where the pattern of mineralization is more likely to be globular than appositional. In certain dental anomalies (e.g., vitamin D-resistant rickets and hypophosphatasia), large areas of interglobular dentin are a characteristic feature.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


