Catalytic sitea
Genesb
Representativec
Catalytic mechanism
Serine
175
Trypsin/chymotrypsin/caspasesd
Residue forms −OH from bound H2O
Threonine
28
Proteasome enzymese
Residue forms −OH from bound H2O
Aspartic acid
21
Pepsin/HIV retropepsin
Aspartyl residue
Cysteine
150
Cathepsins
Cysteinyl residue
Metal ion (Zn2+)
187
Collagenase
Zn2+ ion-bound water moleculef
Table 8.2
Types of zincin metalloproteases in humans
|
Type of enzyme
|
Action
|
Typical enzyme
|
Structural class
|
|---|---|---|---|
|
Exoproteinase
|
Removes N-terminal amino acid
|
Alanyl Aminopeptidasea
|
Metzincin
|
|
Exoproteinase
|
Removes C-terminal amino acid
|
Carboxypeptidase Ab
|
Gluzincin
|
|
Peptidase
|
Removes a C-terminal dipeptide
|
Angiotensin-converting enzymec
|
Gluzincin
|
|
Peptidase
|
Cuts a peptide (<13 aa) internally
|
Neurolysind
|
Gluzincin
|
|
Endoproteinase
|
Cuts a large polypeptide internally
|
Matrilysins, adamalysins and astacinse
|
Metzincin
|
Most metalloproteinases contain a catalytic zinc ion bound to two histidines within a conserved motif, usually HEXXH in the one letter amino acid code where X stands for any amino acid (Fig. 8.1). These enzymes are known as zincins, and they comprise by far the largest clan of metalloendoprotease families. The zincin clan assignments depend on the nature of a third zinc-binding residue: glutamate (E) in gluzincins, aspartate (D) in aspzincins, and histidine or aspartate (H/D) in metzincins. Aspzincins are absent from the human genome (Fig. 8.1) and gluzincins encode proteases other than endoproteases (Table 8.2).
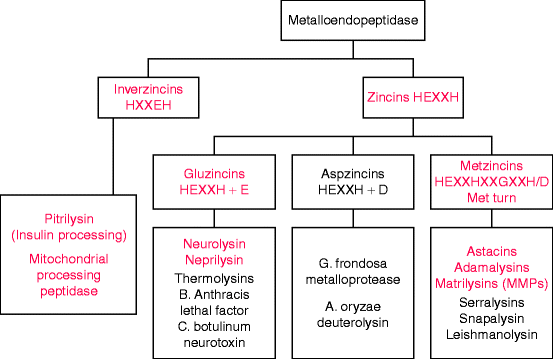

Fig. 8.1
Classification of metallopeptidases by zinc-binding motifs. The major amino acid motif in zincins has two histidine residues that coordinate with the metal ion and a glutamate residue (E) for catalysis. A third residue that coordinates with the metal may be glutamate, aspartate (D), or histidine. In metzincins, the third coordinating residue is histidine or aspartate (H/D), but the name is taken from the presence of a downstream invariant methionine residue (see Fig. 8.2 and text). The red type indicates enzymes or enzyme subfamilies encoded in the human genome (Slightly modified from Fig. 1A of F.X. Gomis-Ruth, “Structural aspects of the metzincin clan of metalloendopeptidases.” Mol. Biotechnol. 24(2):157–202, 2003)
All human metalloendoproteinases are metzincins, named for a downstream methionine residue involved in regulating catalysis by mediating a critical turn that brings an adjacent tyrosine or proline residue close to the catalytic zinc ion. Matrilysins (also called matrix metalloendoproteinases, MMPs) are the major class of metzincin endopeptidases involved in collagen and stromal degradation. The other two classes, adamalysins and astacins (Fig. 8.1), are involved in procollagen processing. Metzincin classes not encoded in the human genome are mostly bacterial virulence factors such as anthrax lethal toxin, a serralysin. Additional metzincins have recently been identified in plants and bacteria.
8.1.1 Catalytic Action of the Metzincin Family
The metzincin catalytic domain consists of a flat surface within a small cleft within which peptide substrates bind and are hydrolyzed (Fig. 8.2a). In astacins, the catalytic domain is stable, but adamalysins require a calcium ion to stabilize the flat surface of the domain above the cleft. Matrilysins require two calcium ions and a second, noncatalytic zinc ion to stabilize this domain (Fig. 8.3). Table 8.3 reviews the roles of specific metal ions that participate in the various stages of collagen processing discussed in this chapter and Chap. 7.
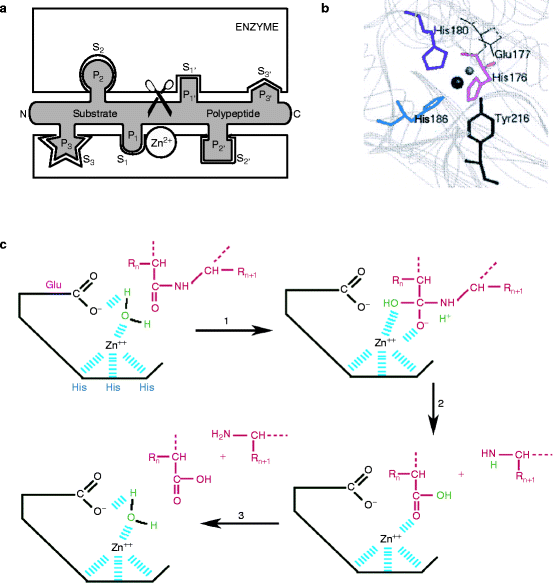
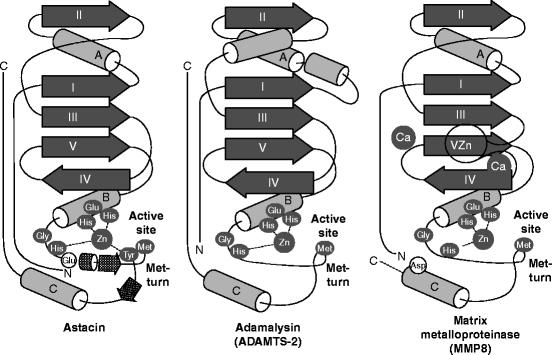

Fig. 8.2
Catalytic metzincin domain. (a) Catalytic endopeptidase subunit showing the peptide binding cleft and surrounding domains. The site binds six amino acids on either side of the bond to be hydrolyzed. Substrate is in an extended conformation and the bound amino acids are numbered (N- to C-terminus): …S3–S2–S1–S1′–S2′–S3′… The products are two peptides: …P3–P2–P1–COOH and NH2–P1′–P2′–P3′… (Slightly modified from Fig. 1B of F.X. Gomis-Ruth, “Structural aspects of the metzincin clan of metalloendopeptidases.” Mol. Biotechnol. 24(2):157-202, 2003). (b) Active site crevice of the metzincin catalytic domain from above. The conserved zinc-binding motif has the structure: His176-Glu177-Xaa-Xaa-His180-Xaa-Xaa-Gly-Xaa-Xaa-His186. The numbering is for serralysin, an astacin family protein, but the relative positions of these amino acids within the motif are identical in all metzincins. The catalytic zinc ion (large sphere) has a coordinated water molecule (small sphere) whose hydrogen atoms are H-bonded to Glu177 (bond shown in Fig. 8.2c). About 20 residues, downstream lies a tyrosine phenolic side chain that points back from beneath the active site as a result of a turn induced by the invariant methionine residue. The ligands involving tyrosine form a distorted trigonal bipyramid in which His176, His186, and the water molecule are equatorial. His180 and Tyr216, respectively, form the upper and lower axial ends (peaks of each pyramid). The Zn2+ ion is approximately 2.2 Å from the histidine ligands, 2 Å from the water molecule, and 2.8 Å from the phenolic O atom of Tyr216. Adamalysins and matrilysins (and some other metzincins) have proline instead of tyrosine at the turn. In these enzymes, a cysteine residue within the pro-domain blocks the cleft by binding to the catalytic zinc ion and excluding water molecule binding (see text) (Modified from Park, H.I. and Ming, L.J. “Mechanistic studies of the astacin-like Serratia metalloendopeptidase serralysin: highly active (>2,000%) Co(II) and Cu(II) derivatives for further corroboration of a “metallotriad” mechanism.” J. Biol. Inorg. Chem. 7(6):600–610. 2002). (c) Mechanism of catalysis. (1) The tyrosine residue moves away from the zinc ion and the water molecule (green) attacks the substrate polypeptide (red) under the influence of the deprotonated glutamate residue. (2) The C-terminal peptide amino group picks up the proton and is released. (3) The remaining hydroxide anion attacks the carboxyl group, which remains held by the zinc ion but it is quickly replaced by another water molecule (Modified from University of Tours, France Web site: http://delphi.phys.univ-tours.fr/Prolysis/introprotease.html)

Fig. 8.3
Architecture of astacins, adamalysin type-2, and matrix metalloproteases. Topology scheme of astacins, adamalysin type-2, and matrix metalloproteinases. The α-helices are shown as rods, β-sheet strands as arrows, and unstructured regions as thin lines. Key amino acids in the catalytic, zinc-binding motif are shown (white in black ellipse). Distinguishing features of each structure are shown as lighter gray for amino acids (black letter), and as unlabeled, secondary-structure elements. In addition to the catalytic zinc ion in all three structures, the calcium ion in adamalysin type-2, and the two calcium ions and second zinc ion in the matrix metalloproteinases (Abbreviated from Fig. 3 in F.X. Gomis-Ruth, “Structural aspects of the metzincin clan of metalloendopeptidases.” Mol. Biotechnol. 24(2):157–202, 2003)
Table 8.3
Metal ions required for collagen processing
|
Metal Ion/s
|
Enzyme/s
|
|---|---|
|
Fe2+
|
Proline/lysine hydroxylase
|
|
Cu1+
|
Lysyl oxidase
|
|
Mg2+/Mn2+
|
Integrin attachment of collagen to cells
|
|
Zn2+/Ca2+
|
Metalloproteases
|
The glutamate (E) residue in the common zincin motif (HEXXH) participates in catalysis by attaching a water molecule that also coordinately bonds to the catalytic zinc ion (Fig. 8.2b). A likely catalytic mechanism involves the loss of a proton from the water molecule, followed by nucleophilic attack of OH−1 on the peptide bond (illustrated in Fig. 8.2c). During this process in astacins such as serralysin, a tyrosine residue flips back and forth during substrate anchoring, cleavage, and product release, in a motion referred to as a “tyrosine switch.” The phenol group of this tyrosine coordinates with the catalytic zinc ion and displaces the water molecule, thus inhibiting catalysis. Interactions of the enzyme away from the catalytic site are transmitted to the tyrosine residue and weaken or strengthen the coordination of its phenolic group to the catalytic zinc ion. In matrilysins and adamalysins, the “tyrosine-switch” is replaced by a “cysteine-switch
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


