Fig. 24.1
Overview of the ventral or inferolateral part of the primary somatosensory cortex (vSI). (a) (b) Lateral view of the cerebral hemisphere of humans (a) and macaques (b). The dark-shaded area roughly indicates the location of the vSI that represents the orofacial structures. The solid line in b corresponds to the section in c. (c) Cytoarchitectonic areas (Brodmann areas 3a, 3b, 1, 2) are shown in a parasagittal section. The broken line indicates layer IV. Dotted lines indicate the boundaries of cytoarchitectonic areas. CS central sulcus, LS lateral sulcus
Non-invasive brain imaging techniques have made significant contributions to our understanding of the brain mechanisms involved in orofacial functions, particularly those specific to humans. However, because of the limitation in spatial and temporal resolution, these techniques are unable to address finer anatomical and physiological details; e.g., the precise location of regions representing each orofacial structure and their neural interconnections, the principles of information coding by individual neurons, and populations of neurons, etc. Therefore, animal studies using nonhuman primates are also of tremendous value as complements to human brain imaging studies, and will continue to be so. In the following sections, we chiefly review the neuroanatomical and neurophysiological studies of the monkey vSI and some relevant neuroimaging studies in humans. Readers may also refer to a related review published recently by another group [9].
24.2 Representation of Orofacial Structures in Area 3b
The primary somatosensory area (SI) consists of three cytoarchitectonic areas, as shown in Fig. 24.1c: Brodmann areas 3 (3a, 3b), 1, and 2. Area 3 is regarded as the primary somatosensory area in a strict sense and is called the “SI proper”, because this area receives the densest projection from the specific somatosensory relay nuclei of the thalamus. Area 3b chiefly represents the contralateral body surface in a mediolaterally elongated band of the cortex. Along this mediolateral axis, the tail, lower limb (foot), trunk, upper limb (hand), face, and oral structures are represented in a somatotopical manner. Our current knowledge of the neuroanatomy of the vSI is based largely on studies performed by Professor Jon H. Kaas (see review [10]) and those of Professor Edward G. Jones. The representations of the hand and face are separated by a histologically visible border in both New [11, 12] and Old World monkeys [13–15]. This “hand-face border” can be detected as a myelin-light septum in brain sections cut parallel to the cortical surface. Further laterally, myelin-dense ovals were shown to indicate anatomical modules that correspond to representations of different orofacial structures in both New [16, 17] and Old World monkeys [13, 15]. In New World monkeys, the myelin-dense ovals are arranged in a rostrocaudal direction and extend to the ventral frontal lobe. There, the lips or chin, teeth (periodontal receptors), and tongue are represented in a caudorostral sequence. Further rostrally, the ipsilateral side of the teeth and tongue are represented. In Old World monkeys, the lips (cheek mucosa), teeth, and tongue are represented in a mediolateral sequence. Further anterolaterally, the ipsilateral side of the teeth and tongue are represented. The ipsilateral representation is a distinctive feature of oral structures, such as the teeth and tongue and each side of the oral structure is represented in area 3b of both hemispheres. This was confirmed in a wide range of primates: prosimian primates, such as the African galago [10], New World monkeys, such as the squirrel monkey and owl monkey [16, 18], Old World monkeys, such as macaques [15, 19, 20], and humans (see review [21]). Such corepresentation of the contralateral and ipsilateral sides of oral structures may facilitate the convergence of input from functionally related portions of both sides (bilateral integration described in the next section).
Dense neural interconnections between representations of different orofacial structures have been documented in both New [17] and Old World monkeys [15]. For example, a recent neuroanatomical study of macaques [15] demonstrated that the tongue representation received dense projections from regions representing the lower and upper teeth and tissue lining the inside of the cheek and lips. Such interconnections may partly explain the presence of neurons having composite receptive fields covering different orofacial structures (described later). In contrast, the hand representation, located medially to the orofacial representation, provided little to no input to either the face or mouth representations in both New [12] and Old World monkeys [14, 15] and it follows that the anatomical hand-face border mentioned earlier is also regarded as a functional border. Another interesting observation may be the relation between the tongue representation and the gustatory related regions [15]: the tongue representation uniquely received projections from areas in the anterior upper bank of the lateral sulcus and anterior insula that may include the primary gustatory area (area G) and other taste-related areas. Also in that study, the tongue representation was likely to receive an additive projection from the lateral surface of the frontal operculum near the lateral sulcus, although the investigators did not particularly emphasize this. This region may presumably correspond to the precentral extension of area 3, which was also implicated in gustatory function [22, 23].
24.3 Neuronal Receptive Fields (RFs) as an Indicator of Hierarchical Information Processing in the vSI
Hierarchical information processing in the vSI was summarized in a previous review [24]. As shown in Fig. 24.2, spatial convergence of somesthetic information arising from orofacial structures proceeds across three cytoarchitectonic areas; i.e., areas 3, 1, and 2 [20, 25], in a manner established in the hand representation (see review [26]). Along this rostrocaudal stream, neuronal receptive fields (RFs) become larger and more complex so that the RFs cover functionally related portions of orofacial structures (composite RFs). The patterns of spatial convergence can be classified into three types (Fig. 24.2): bilateral convergence, intermaxillary convergence, and inter-structural convergence. Furthermore, the spatiotemporal integration also proceeds along this stream: the relative incidence of neurons exclusively responsive to light stroking stimuli (movement-specific neurons) increases moving caudally towards area 2 [27]. Of these, the majority responded with directional selectivity, that is, they responded exclusively to stimuli moving in a particular direction. Most of the movement-specific neurons had ordinally uninterrupted RFs and the remaining had composite RFs discretely covering different structures. The relative incidences of neurons with composite RFs in area 2 were significantly higher in movement-specific neurons than in other neurons, suggesting that the spatiotemporal integration for representing moving objects is accompanied by the convergence of inputs from discrete, but functionally related, oral portions. Such a hierarchical scheme in the vSI might be a prerequisite neural process for dexterous orofacial function and oral stereognosis. The spatial convergence found in awake macaque monkeys was indirectly supported in a subsequent human study using functional MRI [28]. Although neuronal RFs could not be studied in humans, the investigators inspected the degree of activation overlaps between the representations of different oral structures, such as the lips, teeth, and tongue. They showed that the overlap in the middle and caudal portions of the postcentral gyrus was significantly greater than in the rostral portion of the postcentral gyrus.
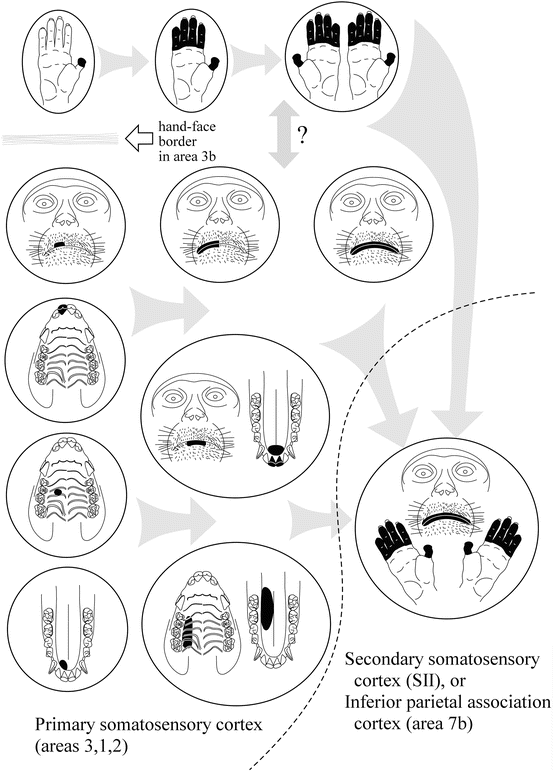

Fig. 24.2
Schematic drawing showing the convergence of somesthetic information across areas of the vSI and adjacent somatosensory-related cortices. Each circle corresponds to a single neuron and its receptive field (RF, drawn in black). Neurons in area 3b are arranged on the left side. Hand, face, and oral structures are represented in a mediolateral sequence. In at least area 3b, the “hand-face border” limits the interconnection between the hand and orofacial representations. Note that actual RF sizes in area 3b neurons are often considerably smaller than depicted here. On moving caudally from area 3b to area 2, neuronal RFs on the hand and orofacial structures become larger and more complex. The patterns of convergence in orofacial neurons can be classified into three types: bilateral convergence, intermaxillary convergence (e.g., upper and lower lips, palate and tongue dorsum), and inter-structural convergence (e.g., tongue tip and anterior teeth). Further caudally in the inferior parietal cortex (area 7b) or laterally in the secondary somatosensory cortex (SII), RFs often cover both the hand and orofacial structures. These neurons in higher-order brain regions are closely related to self-feeding behavior. For further explanation, see the text
The SI receives not only somesthetic inputs arising from the periphery, but also signals from the frontal lobe related to motor command. It is therefore important to determine the activity of vSI neurons during self-generated orofacial movements. The laboratory of Professor B.J. Sessle has played a pioneering role and is making tremendous contributions to this field (for review, see [9]). Their studies on the vSI (chiefly in areas 3b and 1) documented in a trilogy of papers revealed the relations between the neuronal RF properties and neuronal activity during self-generated movements [29–31]. In the first paper [29], monkeys were trained to perform a tongue-protrusion task and biting task. In the tongue protrusion task, a significant alteration of firing rate was observed in ~80 % of tongue RF neurons and 60 % of lip RF neurons. Of note here is that a substantial proportion of neurons did not change their activity during the task, despite apparent orofacial RFs. Moreover, among the task-related neurons, adaptation characteristics of RFs (slowly adapting or rapidly adapting) could not predict the patterns of neuronal activities during the task. For example, task-related neurons with a slowly adapting type of RF did not necessarily fire in a tonic manner during the task: rather, four types of activity patterns; i.e., phasic, tonic, phasic-tonic, and decreased, were detected during the task. In the second paper [30], the monkeys were required to protrude their tongue in each of three directions: the target was positioned at 0°, 30° to the left, or 30° to the right from the midsagittal plane. Again, laterality of a neuronal RF could not predict the preferred tongue-protrusion direction of the neuron. The results of these two papers strongly suggest that neurons with various RF properties are recruited simultaneously even in a simple self-generated orofacial movement. In the third paper [31], electrical or mechanical stimulations were applied to the RFs of each neuron (except to the lingual nerve for tongue RF neurons) when the monkeys were performing the task. Almost all of the neurons tested showed a decrease in evoked activity during the tongue-protrusion task. This finding indicates that disturbing somesthetic inputs arising from the periphery are gated out during self-generated movements. To summarize, the passive properties of neuronal RFs are indeed a reliable indicator for evaluating the flow of sensory information across different brain regions, but those properties alone cannot explain the actual neuronal behavior during self-generated movements.
The bidirectional neural interconnection between the vSI and the ventral primary motor area (vMI) was established in neuroanatomical studies of New [17] and Old World monkeys [15, 32]. In one study on the vMI, most neurons respond to light tactile stimulation rather than stimulation to deep tissues, such as the muscle and joint, which suggests the particular importance of tactile input in motor control [33]. Another important finding in this study was that neurons with RFs on different orofacial structures were intermingled. This may be partly explained by the presence of neurons with composite RFs in the aforementioned vSI and the direct neural projection from the vSI to the vMI. As has been suggested, such complex organization in the vMI may be a prerequisite neural basis for the motor coordination of various structures. The manner of neuroplastic changes in the vMI, as well as vSI, should also be addressed, because such changes permit the acquisition of new motor skills or adaptation to an altered orofacial environment. Studies on this subject were reviewed in detail by Professor B.J. Sessle and colleagues [9, 34].
From the viewpoint of dental pain, vSI neurons that receive input from the tooth-pulp are also important targets of study [35, 36]. In one study, monkeys were trained to detect changes in tooth-pulp stimulus intensity [36]. Some of the neurons examined were implicated in the sensory-discriminative aspect of tooth-pulp sensation, because their discharge rates were correlated with the detection latency of the monkey.
24.4 Hand-Mouth Motor Coordination in Self-Feeding Behavior
Motor coordination of the hand and mouth is essential for self-feeding behavior in primates. There should be neurons that integrate somesthetic information arising from both of the body parts somewhere in the brain; however, this subject is not currently well studied with regard to the vSI. In addition, at least in area 3b, such convergence is considered to be rare, because of the presence of the hand-face border. An earlier study of the vSI reported that several neurons in areas 3b or 1 had discrete RFs on both the radial hand and the lateral part of the face [37]. Since those neurons had face RFs that were remote from the oral slit, they are unlikely to relate to feeding behavior. Rather, neuronal activity related to hand-mouth coordination during self-feeding was documented in higher order cortical areas other than the vSI (Fig. 24.2
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


