Fig. 3.1
Composition of the connective tissue extracellular matrix. Collagen fibers, maroon: Elastic fibers, green: Hyaluronan-proteoglycan matrix, gray. Fibroblasts, a macrophage, a mast cell, and a capillary containing a red blood cell are also shown (Modified from Fig. 19–34 in The Molecular Biology of the Cell. B. Alberts et al., 4th Ed. 2002. Garland Science, Taylor & Francis Group, NY)
Where a stromal surface meets the epithelium, the fibroblasts interact with epithelial cells to form a basal lamina. This thin, flexible layer of specialized extracellular matrix (40 to 120 nm thick) underlies all epithelial cell sheets or tubes and is detected by visualizing the tissue under an electron microscope. Figure 3.2 shows three alternative organizations of a basal lamina: surrounding skeletal muscles, underlying epithelia, and interposed between two cell sheets as in the kidney glomeruli. A basal lamina acts as a semipermeable membrane, to separate specialized cells such as epithelial or muscle cells from soluble stromal proteins, or in the case of the kidneys, to act as a membrane through which metabolic end products from the body can pass. The rich capillary network immediately beneath an epithelium ensures that essential nutrients and low molecular weight cell-modifying agents (chemokines) have access to the overlying cells. Basal laminas prevent soluble plasma proteins from passing into stromal fluid or soluble stromal proteins from passing into epithelial fluid.
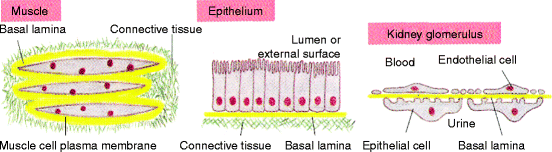

Fig. 3.2
Relationship of the connective tissue stroma to an epithelium. Fibroblasts secrete unique types of laminin proteins that interact with each other and type IV collagen, to form a basal lamina (yellow) that is tightly attached to the connective tissue (stromal) cells and also to the associated muscle or epithelial cell. The kidney glomerulus is a specialized tissue in which the stroma is absent and endothelial cells and epithelial cells are separated by the basal lamina which filters the blood as the first step in urine collection. The attachment of an epithelium to stromal collagen is discussed in detail in Chap. 5 (Fig. 19-55 in The Molecular Biology of the Cell. B. Alberts et al., 4th Ed. 2002. Garland Science, Taylor & Francis Group, NY)
3.2 Collagen
Collagen is the major protein of the stroma. There are two major groups of collagens encoded in the genome: fibrillar and non-fibrillar (Table 3.1). Collagen fibers are the most abundant group and visible to the naked eye. Collagen fibrils are visible only through a light microscope and collagen microfibrils or filaments only though an electron microscope. In addition to forming and maintaining tissue integrity and stability, collagens also form a bioactive surface that regulates cell differentiation, morphogenesis, and migration, as well as, wound repair, inflammation, and thrombosis. In addition, alterations in collagen metabolism lead to a large spectrum of diseases, including common disorders like osteoarthritis and atherosclerosis. An equal number of other genes encode collagen-like domains in various other proteins such as the complement proteins of blood plasma (see Sect. 3.3.2.).
Table 3.1
Major functions, genetic, and polypeptide composition of some common types of collagen
|
Collagen functions and types
|
Gene name
|
Polypeptide composition
|
|---|---|---|
|
FIBRILLAR collagens (Types I, II, III, V, & XI)
|
COL1A1, COL1A2
|
[α1(I)]2[α2(I)] (Most common)
|
|
COL2A1
|
[α1(II)]3
|
|
|
Dermal reticular fibers (Type III with V)
|
COL3A1
|
[α1(III)]3
|
|
COL5A1, COL5A2
|
[α1(V)]2[α2(V)]
|
|
|
COL11A1, COL11A2
|
[α1(XI)]2[α2(XI)]
|
|
|
NETWORK (fine bone marrow/epiphyseal reticular fibers) (Type III with VIII & X)
|
COL8A1, COL8A2
|
[α1(VIII)]2[α2(IV)]
|
|
COL10A1
|
[α1(X)]3
|
|
|
BASEMENT MEMBRANE collagens (Type IV)
|
COL4A1, COL4A2, COL4A3
|
[α1(IV)]2[α2(IV)] (In all tissues)
|
|
COL4A4, COL4A5, COL4A6
|
[α3(IV)][α4(IV)][α5(IV)] (Glomerulus)
|
|
|
[α5(IV)]2[α6(IV)] (Restricted)
|
||
|
MICROFIBRILLAR collagens (Type VI)
|
COL6A1, COL6A2, COL6A3
|
[α1(VI)][α2(VI)][α3(VI)]
|
|
ANCHORING fibrils with interrupted triple helix; long chain collagen (Type VII & XVII)
|
COL7A1, COL17A1
|
[α1(VII)]3 [α1(XVII)]3
|
|
FACIT Fibril-associated collagens with interrupted triple helices (Types IX & XII)
|
COL9A1, COL9A2, COL9A3
|
[α1(IX)][α2(IX)][α3(IX)]
|
|
COL12A1
|
[α1(XII)]3
|
The fibrillar collagens are made from types I, II, III, V, and XI polypeptides (Table 3.1). The most predominant collagen is type I, but mixtures with other collagen types affects the fiber structure. Type I fibers are often found as a complex with type V fibers for various reasons: to facilitate corneal transparency; to limit fiber thickness during tissue repair and to help form the architecture of various collagen-containing tissues such as tendons or the placenta. Type II fibers, which are unique to cartilage, form a complex with type XI collagen to limit thickness and enhance binding to proteo-glycosaminoglycans. Reticular fibers are delicate fibers composed mainly of type III collagen and extensively covered with glycosaminoglycans and glycoproteins. In the dermis of the skin and gingiva, the reticular fibers extend out from type I collagen fibers that have already associated with type V polypeptides. Within the bone marrow and other less fibrous tissues, the type III collagen of reticular fibers associates with a mixture of non-fibrillar type VIII and type X polypeptides.
All collagen fibers, fibrils and microfibrils have an alternating light and dark staining pattern (striated or banding pattern) that has a characteristic appearance (upper half of Fig. 3.3). This pattern is due to the staggered array of tropocollagen triple helices (lower half of Fig. 3.3, also discussed in Sects. 4.2.1, 8.2.1 and 8.3.4). The fibrillar collagens are synthesized as a precursor, procollagen. On secretion, the N-terminal and C-terminal domains are removed. Sequences identical or similar (homologous) to the N-terminal procollagen domain of the commonly found fibrillar collagens are expressed on other proteins and are referred to as procollagen domains.
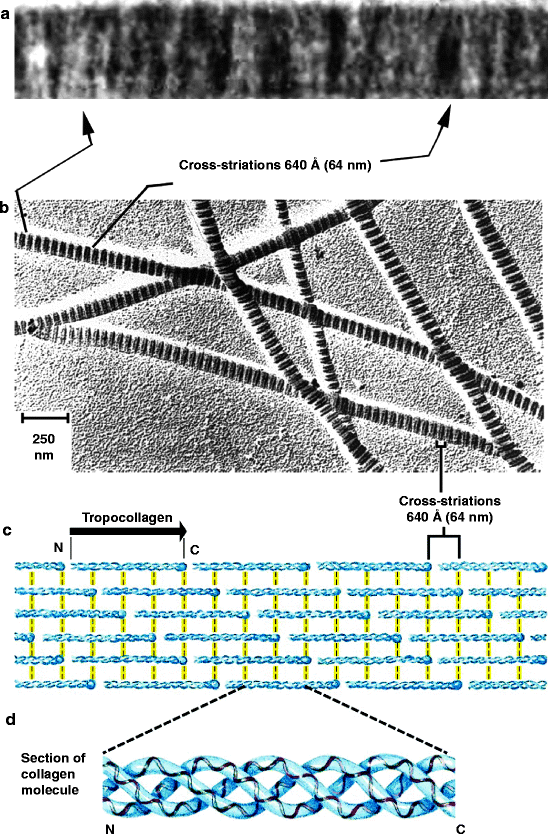
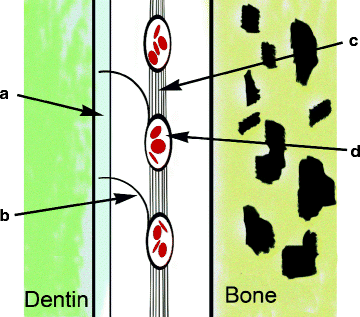

Fig. 3.3
The striated appearance of collagen fibers. Collagen fibrils are made up of tropocollagen molecules aligned in a staggered fashion and cross-linked for strength. (a) Electron micrograph showing cross-striations from native rat tail collagen. Alternate, broad, mainly light and mainly dark bands with a period of approximately 64 nm are prominent (From Fig. 1 of Cox RW, Grant RA and Kent CM (1972) “The interpretation of electron micrographs of negatively stained native collage.” Journal of Cell Science 10:547–554). (b) Less magnified view of collagen fibers. (c) Self-aggregation of tropocollagen. The N– and C-terminal ends of tropocollagen are called telopeptide domains and they interact with adjacent tropocollagen molecules (yellow lines). (d) Tropocollagen self-aggregating unit. A tropocollagen molecule is a self-aggregating unit of three long polypeptides that are intertwined in a triple helix. It is cut out from a genetically encoded polypeptide called procollagen as described in Sect. 4.2.1 (Figs. (b)–(d) modified from Fig. 4–13 in Lehninger Principles of Biochemistry. D.L. Nelson and M.M. Cox, 4th Ed. 2005. W.H. Freeman & Co., NY)

Fig. 3.4
An oxytalan fiber tract. (a) Cementum; (b) Principal oxytalan fiber; (c) Oxytalan tract; (d) Periodontal vessel (From Fig. 2.4, Oxytalan fibers forming a network that attaches blood vessels to the cementum. In article titled, “Force Generation and Reaction within the Periodontium.” Caputo AA and Wylie RS: UCLA School of Dentistry website; http://www.dent.ucla.edu/pic/members/force/index.html) Figure modified slightly and redrawn by Dr Wirsig-Weichmann. Permission granted by the Authors
The kidney contains small amounts of fibrillar collagen, but its major collagen is a non-fibrillar network collagen (type IV), a part of basal laminas called the lamina densa (Sect. 5.1.1). Basal laminas are especially well developed in the kidney as exemplified in the glomerulus (Fig. 3.2). The collagen contents of various tissues are indicated in Table 3.2 and their fiber arrangements in Table 3.3.
Table 3.2
Collagen and elastin content of some tissues (g/100 g of dry weight)
|
Tissue
|
Collagen
|
Elastin
|
|---|---|---|
|
Ligament
|
17
|
50
|
|
Aorta
|
12–24
|
30–57
|
|
Tendon (achilles)
|
86
|
4
|
|
Dermis of skin
|
72
|
2–5
|
|
Liver
|
3.9
|
0
|
|
Cartilage
|
46–63
|
0
|
|
Cornea
|
68.1
|
0
|
|
Bone, mineral free
|
88
|
0
|
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


