This article discusses the radiographic manifestation of jaw lesions whose etiology may be traced to underlying systemic disease. Some changes may be related to hematologic or metabolic disorders. A group of bone changes may be associated with disorders of the endocrine system. It is imperative for the clinician to compare the constantly changing and dynamic maxillofacial skeleton to the observed radiographic pathology as revealed on intraoral and extraoral imagery.
Key points
- •
Perform a detailed medical history and physical examination for each patient.
- •
Understand the potential osseous changes that may be caused by an underlying medical problem.
- •
Understand normal radiographic anatomy.
- •
Identify and describe any osseous changes noted on radiographic imagery of the patient.
- •
Develop a differential diagnosis and treatment plan.
This article is written to highlight and focus on patients with medical conditions seeking dental treatment. History and physical examination findings combined with laboratory and imaging findings lead the clinician to understand the burden of the underlying systemic condition and its effect on the impending dental treatment. It is the responsibility of the dental practitioner to be aware of these systemic conditions, diagnose and appropriately refer to a specialist when needed, and more commonly, manage them as dental patients. Any pertinent data must be used in conjunction with the radiographic investigation or other imaging modality to arrive at a clinical diagnosis. The systemic conditions that are covered range from endocrine abnormalities and developmental conditions to malignancies that have skeletal manifestations. Some of the systemic conditions that may affect the dental treatment or outcomes are described elsewhere in this issue.
Sickle cell anemia
Introduction
Sickle cell disease (SCD) comes from a specific form of anemia that is the result of homozygosity for the mutation that causes sickling of hemoglobin (HbS). Sickle cell anemia also has been referred to as HbSS, SS disease, and hemoglobin S. In the heterozygous populations that have only one sickle gene and one normal adult hemoglobin gene, the condition is referred to as sickle cell trait or HBAS. Other forms of sickle disease includes sickle cell hemoglobin c (HbSC), sickle beta plus-thalassemia (HbS/β + ), and sickle beta zero-thalassemia (HbS/β 0 ) According to the Centers for Disease Control and Prevention (CDC data) that looked into the data from 44 states representing 88% of population, the prevalence of SCD was approximately 73 per 1000 births among African American individuals and 7 per 1000 among Hispanic American individuals. The overall rate was approximately 15 per 1000 live births in the United States.
Summary of Clinical Features
Anemia, infection, and vaso-occlusion are the most common reasons for the complications related to SCD, episodes of pain in the chest, back, abdomen, or extremities. Multiple areas are often involved simultaneously. The acute chest syndrome leading to respiratory insufficiency will have the cardinal features of fever, pleuritic chest pain, referred abdominal pain, cough, lung infiltrates, and hypoxia. Patients with SCD now survive into their fifth or sixth decades in the industrialized countries.
Radiographic Features
The vaso-occlusion leads to bone infarcts, osteomyelitis, orbital wall infarction, and subperiosteal hemorrhage. Chronic anemia leads to internal carotid artery stenosis and extramedullary hematopoiesis. The infections lead to osteomyelitis and regional lymphadenopathy. Bone involvement is the most common clinical feature of SCD, mainly the long bones and to a lesser extent the maxillofacial area, due to the small number of marrow spaces within these bones. In the head and neck, the most frequently reported location is the orbital wall, followed by the mandible and skull base. There are both acute and chronic phases of bone involvement; in the acute phase, vaso-occlusive crisis and osteomyelitis are notable, whereas in the chronic phase, bone marrow hyperplasia, osteoporosis, and iron deposition in the marrow due to repeated transfusions may be noted. Avascular necrosis of the bone is a major problem in the long bones, especially the hip, although it is not a common occurrence within the jaws.
Imaging Protocols
Bone infarction leading to change in the trabecular pattern may be a radiographic sign noted in patients with SCD. MRI is a much more sensitive imaging technique over computed tomography (CT) for these changes.
Imaging Findings/Pathology
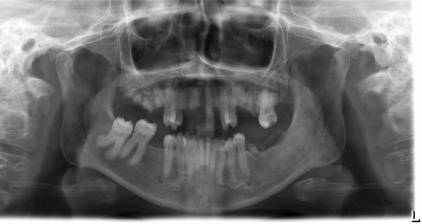
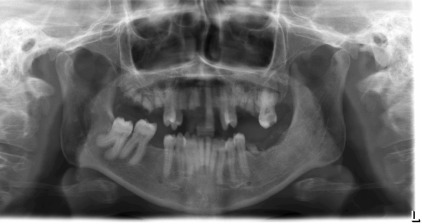
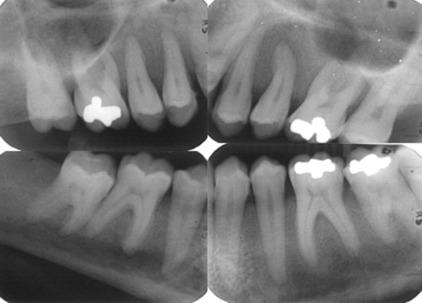
Radiographically, cortical defects, adjacent soft tissue fluid collection that communicates with the medullary compartment through cortical defects, and ill-defined bone marrow enhancement are indicative of osteomyelitis. A panoramic radiograph ( Fig. 1 ) of a 34-year-old woman with compensated SCD, who presented to the oral medicine admissions clinic with severe abdominal distension and vasculitis affecting the lower extremities showed inconsistent trabecular changes, enlarged bone marrow spaces, especially in the lower anterior region, and some cortical defects. Hypercementosis of some teeth was noted as an incidental finding.
Differential Diagnosis
- •
Sickle cell anemia
- •
Thalassemia
- •
Spherocytosis
- •
Elliptocytosis
- •
Iron deficiency anemia
Summary
It is important to remember that a reduction of the number of trabeculae, a generalized rarefaction accompanied by some thinning of the mandibular cortical plates, may be indicative of SCD.
Sickle cell anemia
Introduction
Sickle cell disease (SCD) comes from a specific form of anemia that is the result of homozygosity for the mutation that causes sickling of hemoglobin (HbS). Sickle cell anemia also has been referred to as HbSS, SS disease, and hemoglobin S. In the heterozygous populations that have only one sickle gene and one normal adult hemoglobin gene, the condition is referred to as sickle cell trait or HBAS. Other forms of sickle disease includes sickle cell hemoglobin c (HbSC), sickle beta plus-thalassemia (HbS/β + ), and sickle beta zero-thalassemia (HbS/β 0 ) According to the Centers for Disease Control and Prevention (CDC data) that looked into the data from 44 states representing 88% of population, the prevalence of SCD was approximately 73 per 1000 births among African American individuals and 7 per 1000 among Hispanic American individuals. The overall rate was approximately 15 per 1000 live births in the United States.
Summary of Clinical Features
Anemia, infection, and vaso-occlusion are the most common reasons for the complications related to SCD, episodes of pain in the chest, back, abdomen, or extremities. Multiple areas are often involved simultaneously. The acute chest syndrome leading to respiratory insufficiency will have the cardinal features of fever, pleuritic chest pain, referred abdominal pain, cough, lung infiltrates, and hypoxia. Patients with SCD now survive into their fifth or sixth decades in the industrialized countries.
Radiographic Features
The vaso-occlusion leads to bone infarcts, osteomyelitis, orbital wall infarction, and subperiosteal hemorrhage. Chronic anemia leads to internal carotid artery stenosis and extramedullary hematopoiesis. The infections lead to osteomyelitis and regional lymphadenopathy. Bone involvement is the most common clinical feature of SCD, mainly the long bones and to a lesser extent the maxillofacial area, due to the small number of marrow spaces within these bones. In the head and neck, the most frequently reported location is the orbital wall, followed by the mandible and skull base. There are both acute and chronic phases of bone involvement; in the acute phase, vaso-occlusive crisis and osteomyelitis are notable, whereas in the chronic phase, bone marrow hyperplasia, osteoporosis, and iron deposition in the marrow due to repeated transfusions may be noted. Avascular necrosis of the bone is a major problem in the long bones, especially the hip, although it is not a common occurrence within the jaws.
Imaging Protocols
Bone infarction leading to change in the trabecular pattern may be a radiographic sign noted in patients with SCD. MRI is a much more sensitive imaging technique over computed tomography (CT) for these changes.
Imaging Findings/Pathology
Radiographically, cortical defects, adjacent soft tissue fluid collection that communicates with the medullary compartment through cortical defects, and ill-defined bone marrow enhancement are indicative of osteomyelitis. A panoramic radiograph ( Fig. 1 ) of a 34-year-old woman with compensated SCD, who presented to the oral medicine admissions clinic with severe abdominal distension and vasculitis affecting the lower extremities showed inconsistent trabecular changes, enlarged bone marrow spaces, especially in the lower anterior region, and some cortical defects. Hypercementosis of some teeth was noted as an incidental finding.
Differential Diagnosis
- •
Sickle cell anemia
- •
Thalassemia
- •
Spherocytosis
- •
Elliptocytosis
- •
Iron deficiency anemia
Summary
It is important to remember that a reduction of the number of trabeculae, a generalized rarefaction accompanied by some thinning of the mandibular cortical plates, may be indicative of SCD.
End-stage renal disease and renal osteodystrophy
Introduction
End-stage renal disease (ESRD) may cause a skeletal change known as renal osteodystrophy. The kidney disease results in decreased levels of 1,25-dihydroxy vitamin D, which is responsible for the active transport of calcium in the small bowel. These patients may present with hypocalcemia as a result of compromised calcium absorption and hyperphosphatemia resulting from reduction in renal phosphorus excretion. Secondary hyperparathyroidism may be a consequence of sustained serum calcium stimulating the parathyroid glands to produce parathyroid hormone (PTH).
Imaging Findings/Pathology
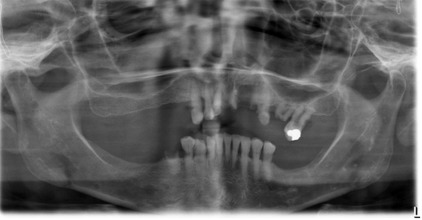
The radiographic features of renal osteodystrophy are quite variable. Some changes of the skeleton resemble those changes observed in rickets, and other changes are consistent with hyperparathyroidism, including generalized loss of bone density and thinning of bony cortices ( Fig. 2 ). There may be occasional findings of brown tumors, similar to those seen in primary hyperthyroidism. The occasional size increase of the mandible is due to enlargement of the cancellous bone component that has a dense granular trabecular pattern. In renal osteodystrophy, the density of the mandible and maxilla may be less than normal and intermittently may be greater than normal. Manifestations include a decrease or an increase in the number of internal trabeculae, and the trabecular bone pattern may be granular. The cortical boundaries may be thinner or less noticeable. It is important to note that these bone changes may persist after a successful renal transplant because of hyperplasia of the parathyroid glands, resulting in a continued elevation of PTH. The lamina dura may be absent or not as noticeable on occasion.
Differential Diagnosis
- •
Osteomalacia and renal osteodystrophy
- •
Osteoporosis
- •
Multiple myeloma
- •
Osteomalacia and renal osteodystrophy
- •
Sickle cell anemia
Summary
Loss of calcium and vitamin D promotes demineralization of the skeleton (referred to as renal osteodystrophy). Risk of fractures increases with prolonged and uncontrolled secondary hyperparathyroidism and results in increased complications that result from fractures. Bone densitometry can be used to detect reduced bone density and monitor its response to treatment. Prevention or reversal of this complication can be achieved through regulation of parathyroid hormone, vitamin D, and calcium levels.
When treating an patient with ESRD or those on hemodialysis, expect to observe a generalized bony rarefaction when interpreting intraoral and extraoral images of the head and neck. Also, you should relate these finding to recent dialysis blood draws for osteodystrophic panels, as well as complete metabolic panels.
Hyperparathyroidism
Hyperparathyroidism is an endocrine abnormality in which there is an excess of circulating PTH. An excess of serum PTH increases bone remodeling in preference of osteoclastic resorption, which mobilizes calcium from the skeleton. In addition, PTH increases renal tubular reabsorption of calcium and renal production of the active vitamin D metabolite 1,25(OH)2D. The net result of these functions is in an increase in serum calcium levels. Primary hyperparathyroidism usually results from a benign tumor (adenoma) of 1 of the 4 parathyroid glands, resulting in the production of excess PTH. Infrequently, some individuals may have hyperplastic parathyroid glands that secrete excess PTH. The combination of hypercalcemia and an elevated serum level of PTH is diagnostic of primary hyperparathyroidism.
Secondary hyperparathyroidism results from an increase in the production of PTH in response to hypocalcemia. The underlying hypocalcemia may result from an inadequate dietary intake or poor intestinal absorption of vitamin D or from deficient metabolism of vitamin D in the liver or kidney. This condition produces clinical and radiographic effects similar to those of primary hyperparathyroidism. PTH is produced and secreted by the parathyroid glands, whose activity is controlled by the free (ionized) serum calcium level. Increased PTH secretion results in a condition called hyperparathyroidism. Hyperparathyroidism is divided into primary, secondary, and tertiary types. Primary hyperparathyroidism is characterized by elevated PTH secretion. This is a result of an abnormality in one or more of the parathyroid glands. Adenomas are the main cause in approximately 85% of primary hyperparathyroidism cases. Most cases of primary hyperparathyroidism are identified by the presence of hypercalcemia and hypophosphatemia on routine multipanel serum analysis. Secondary hyperparathyroidism is caused by hypocalcemia or vitamin D deficiency acting as a stimulus for excessive PTH production. Chronic renal failure is the main cause of secondary hyperparathyroidism. It results in hypocalcemia and the parathyroid glands hyperactivity to compensate for this low serum calcium level.
Radiographic Features
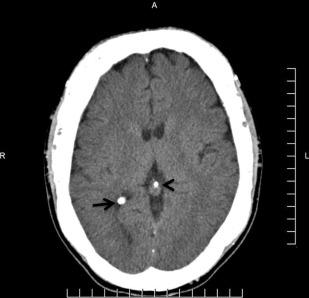
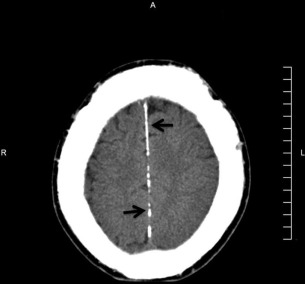
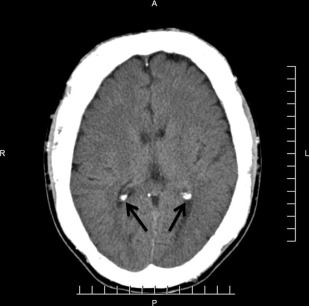
The principal radiographic change is calcification of the basal ganglia. On skull radiographs, this calcification appears flocculent and paired within the cerebral hemispheres on the posteroanterior (PA) view. Radiographic examination of the jaws may reveal dental enamel hypoplasia, external root resorption, delayed eruption, or root. The important radiographic presentation of hyperparathyroidism in the dental radiographs is the loss of lamina dura ( Fig. 3 ), ground glass appearance of the bone, thinning of the mandibular cortex, and, in established cases, gives the appearance of “floating teeth” within the bone due to generalized loss of lamina dura. Intracranial calcifications noted in a patient with exuberant hyperparathyroidism secondary to long-standing ESRD are shown. Note the generalized thickening of skull vault ( Figs. 4–6 ).
Differential Diagnosis
The following can be considered in the differential diagnosis:
- •
Hyperparathyroidism
- •
Central giant cell granuloma
- •
Fibro-osseous lesion
Summary
Panoramic radiographs, CT scans, ultrasonography, and parathyroid scintigraphy with 99m Tc were all useful radiographic methods for the correct diagnosis if serum analysis is suggestive of underlying disease.
Osteoporosis
Introduction
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a subsequent increase in bone fragility and susceptibility to fracture. Osteoporosis is identified internationally, affecting mostly old and middle-aged women. An imbalance occurs in bone resorption and formation. Decrease in bone formation results in changes in trabecular architecture, the volume of trabecular bone, and the size and thickness of individual trabeculae. Osteoporosis occurs with the aging process of bone and can be considered a variation of normal (primary osteoporosis). By the third decade of life, there is a gradual and progressive decline, occurring at the rate of approximately 8% per decade in women and 3% per decade in men. The loss of bone mass with age is so gradual that it is virtually imperceptible until it reaches significant proportions. Secondary osteoporosis may result from nutritional deficiencies, hormonal imbalance, inactivity, or corticosteroid or heparin therapy.
Imaging Findings/Pathology
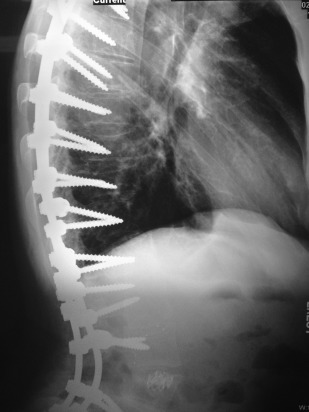
Osteoporosis results in an overall reduction in the density of bone ( Fig. 7 ). This reduction may be observed in the jaws by using the unaltered density of teeth as a comparison. There may be evidence of a reduced density and thinning of cortical boundaries, such as the inferior mandibular cortex ( Fig. 8 ). Reduction in the volume of cancellous bone is more difficult to assess, although new techniques to analyze the trabecular pattern in intraoral images are being developed. Reduction in the number of trabeculae is least evident in the alveolar process, possibly because of the constant stress applied to this region of bone by the teeth. On occasion, the lamina dura may appear thinner than normal. In other regions of the mandible, a reduction in the number of trabeculae may be evident. Accurate assessment of bone mass loss is difficult, but may be done with sophisticated techniques, such as dual-energy photon absorption or quantitative CT programs.
Diagnostic Criteria
Dual-energy X-ray absorptiometry is considered the standard for measurement of bone density; the World Health Organization considers bone mineral density measurements diagnostic for osteoporosis. Certain features may be observed on a panoramic image. A constellation of thinning of the mandibular cortex, external oblique ridges, and generalized rarefaction might represent an osteoporotic patient.
Differential Diagnosis
- •
Hyperparathyroidism
- •
Osteomalacia and renal osteodystrophy
- •
Homocystinuria/homocysteinemia
- •
Multiple myeloma
- •
Paget disease
- •
Sickle cell anemia
- •
Scurvy
Summary
We in the dental profession have the opportunity to use intraoral and extraoral imaging technology inherent to our field of patient care to identify osteoporotic changes in the maxillofacial skeleton that may influence patient care. Of utmost importance is the current use of nitrogen-bearing bisphosphonate drugs that carry with them the potential for antiresorptive osteonecrotic jaw disease.
Cushing syndrome
Cushing syndrome is caused by the clinical manifestation of pathologic hypercortisolism. These excess glucocorticoids can be from increased endogenous production or prolonged exposure to exogenous use of glucocorticoid products. Although endogenous Cushing syndrome is a rare disease, drug-related or exogenous Cushing syndrome from glucocorticoid products is commonly seen in clinical practice. This section focuses on iatrogenic, or drug-related, Cushing syndrome.
Drugs that have been reported to result in hypercortisolism are glucocorticoids, megestrol acetate, and herbal preparations that contain glucocorticoids.
Individuals with Cushing syndrome can develop moon facies, facial plethora, supraclavicular fat pads, buffalo hump, truncal obesity, and purple striae.
Individuals often experience proximal muscle weakness, easy bruising, weight gain, hirsutism, and pediatric growth retardation. Hypertension, osteopenia, diabetes mellitus, and impaired immune function also may occur. Additionally, subclinical hypercortisolism may be more common than is generally recognized in patients with osteoporosis in whom secondary causes of osteoporosis have been excluded.
Imaging Findings/Pathology
Cushing syndrome, which cannot be differentiated radiologically from that of the natural form of the disease, has been frequently described. It is liable to occur with a cortisone or cortisone-equivalent dosage of 50 to 100 mg a day over periods varying from 6 months to 3 years. Osteoporosis, fractures, and joint degenerations may be observed in the absence of the fully developed picture of Cushing syndrome. Such changes may occur after much shorter periods of steroid therapy.
Temporomandibular joint diseases of systemic origin
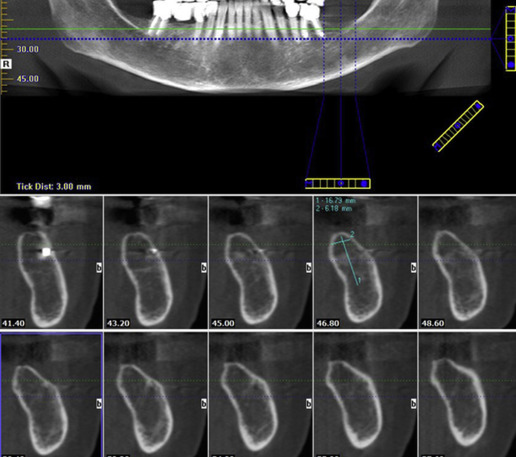
The research diagnostic criteria that are universally accepted for the diagnosis of temporomandibular disorder (TMD) are quite stringent in their categorization of clinical and radiographic findings. Only those cases of osteoarthritis in which there is clear-cut deformation of the condyles due to subcortical cyst, surface erosions, osteophytosis, or generalize sclerosis were classified into the category C (the most severe form), the other 2 categories, A and B, being less severely involved and imaging findings are within normal limits. These patients constitute a large majority of the patient population in which the abnormality is mostly soft tissue or disk-related in origin for which standard radiography is of no use. A magnetic resonance examination of the joints might show the changes related to the disk and whether or not it is displaced.
The imaging of the temporomandibular joint (TMJ) may be indicated based on the patient’s signs and symptoms and examination findings. Clinical examination finding suggestive of joint restriction or progressive trismus should alert the clinician of an impending TMD, and extraoral imaging is indicated in such situations ( Fig. 9 ). On the other hand, incidental findings may be observed on panoramic, cone beam CT (CBCT), and lateral and PA cephalometric and skull views acquired during the examination for other procedures. This patient population may be treated with drugs such as corticosteroids, which of course may also contribute to osteoporotic changes in the bone, and antineoplastic drugs, such as methotrexate, which have the potential of causing severe bone marrow suppression.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses