Chapter 3
Supragingival Microbes
Abstract
This chapter describes the cell and colony morphology, cultural and biochemical characteristics of oral microbes that are mainly isolated from supragingival plaques, including gram-positive bacteria of the genera Actinomyces, Bifidobacterium, Lactobacillus, Rothia, Staphylococcus, and Streptococcus, as well as gram-negative bacteria of the genera Leptotrichia and Veillonella.
Keywords
Actinomyces; Bifidobacterium; Lactobacillus; Leptotrichia; Rothia; Staphylococcus; Streptococcus; Supragingival; Veillonella
3.1. Gram-positive bacteria
3.1.1. Actinomyces
Actinomyces are irregular gram-positive bacilli and are also commonly found anaerobic bacteria in oral samples. When they were first discovered, actinomycetes were believed to be fungi or were grouped as “other microorganism.” Recently, numerous studies have shown that actinomycetes have general characteristics common to bacteria and were classified as prokaryotic organisms. In the 1984 edition of Bergey’s Manual of Determinative Bacteriology, Actinomyces were included in the group of gram-positive irregular bacilli. Common members of the Actinomyces genus in oral microbiology are Actinomyces israelii, Actinomyces naeslundii, Actinomyces odontolyticus, and Actinomyces viscosus. They are characterized by a relatively high GC content in their DNA, ranging from 57% to 69% (Tm method). The type species of this genus is Actinomyces bovis.
The shape and size of bacterial cells can vary greatly but are often found to be irregular branched bacilli with a diameter of 0.2–1.0 μm and a length of about 5.0–10.0 μm. The cells are usually rod-shaped, but can occasionally be club-shaped with irregular arrangements including single, paired, chain, clusters, and fence-shaped. The cells produce no spores, show no motility, and also do not produce conidia. The main distinguishing characteristic of Actinomyces cells is that the cell wall does not contain DAP and glycine.
There are differences at the species level regarding oxygen sensitivity, but a primary culture of Actinomyces requires an anaerobic environment. Spider-like microcolonies or branched mycelia can form on agar plates after 18–24 h incubation. These typical spider web-shaped colonies can help identify the genus of bacteria.
All strains of actinomycetes can ferment glucose and fructose to produce acid, without producing gas. Other tests, including fermentation of raffinose, xylose, cellobiose, laetrile, ribose, salicin, catalase production, reduction test using nitrate and nitrite, urea hydrolysis, or gelatin, can help identify different species.
Actinomycetes are normal members of the oral flora and are the dominant bacteria in dental plaque. A. israelii, A. naeslundii, A. odontolyticus, and A. viscosus can be detected in human dental plaque, dental calculus, and saliva. The main colonization site of Actinomyces mai is in the gingival sulcus. Clinical and epidemiological investigations indicate that A. israelii can cause actinomycosis, conjunctivitis, lachrymal and other diseases of the face, neck, lung, and abdomen. A. naeslundii and A. mai can be detected in clinical samples of gingivitis, periodontitis, pulp periapical infection, and pericoronitis. A. viscosus is suspected to be a cariogenic bacterium.
3.1.1.1. Actinomyces israelii
A. israelii are gram-positive irregular bacilli (Figure 3.1(A)–(C)). The culture atmosphere requires CO2, as the culture grows poorly or not at all under ordinary atmospheric environment. Bacterial growth can be inhibited by 4–6% NaCl, 20% bile, or 0.005% crystal violet. Characteristic colonies are shown in Figure 3.1(D)–(F). The DNA GC content ranges from 57% to 65% when analyzed using the Tm method. The type strain is ATCC12102 (WVU46, CDCX523, W855).
The main habitat of this species is the oral cavity. A. israelii often colonizes the tonsils and plaque but can also be detected in the human gut and the female reproductive tract. This bacterium is mainly a pathogen of the face and neck, and causes lung and abdominal actinomycosis. It can also infect the lachrymal sac and conjunctiva. It is detected in oral mixed infections such as gingivitis, periodontitis, and pericoronitis, but the link between A. israelii and the infections is unclear.
Young colonies of A. israelii are typical filamentous microcolonies. Mature colonies are characteristically less than 2 mm in diameter, raised, white, opaque, and molar-shaped. The species shows no β-hemolytic reaction on blood agar.
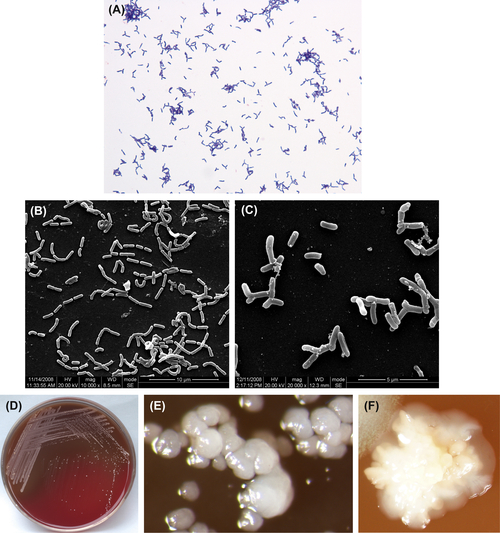
Figure 3.1 (A) Actinomyces israelii cells (Gram stain). (B) A. israelii cells (SEM). (C) A. israelii branch cells (SEM). A. israelii always display branching rods and short filamentous with no spores, Gram stain negative. (D) A. israelii colonies (BHI blood agar). (E) A. israelii molar-shaped colonies (stereomicroscope). (F) A. israelii colonies detached from plaque samples (stereomicroscope).
3.1.1.2. Actinomyces naeslundii
A. naeslundii is a gram-positive irregular bacillus (Figure 3.2(A) and (B)). Under aerobic conditions without CO2, the culture may not grow. However, cultures can be grown in an anaerobic environment without CO2. Some strains can be grown at 45 °C. Growth can be inhibited by 6% NaCl. Characteristic colonies are shown in Figure 3.2(C) and (D), and broth culture is shown in Figure 3.2(E). The DNA GC content is approximately 63–69% using the Tm method. The type strain is ATCC12104 (NCTC10301, WVU45, CDCX454).
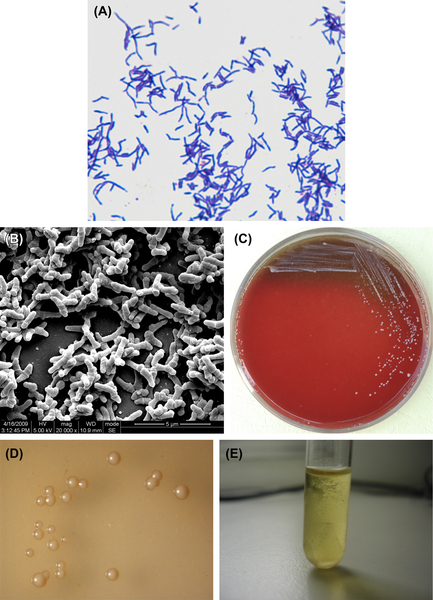
Figure 3.2 (A) Actinomyces naeslundii cells (Gram stain). (B) A. naeslundii cells (SEM). A. naeslundii cells are mainly irregular branched rods or short filaments without spores. The cells are gram-negative. (C) A. naeslundii colonies (BHI blood agar). (D) A. naeslundii colonies (stereomicroscope). (E) A. naeslundii liquid culture (BHI broth).
A. naeslundii is a member of the normal human oral flora and can be found on the tonsils and in dental plaque. It can take part in mixed bacteria infections and is one of the pathogenic bacteria in root caries. It is often detected in clinical specimens of periodontitis or infected root canals, but the pathogenesis is unclear. This bacterium can lead to actinomycosis at many locations such as face, neck, chest, abdomen, and eye, and can cause infection of the female genital tract and knee joint empyema.
Young colonies of A. naeslundii can appear as filiformed microcolonies. Mature colonies are convex or flat, rough or smooth without center sag. The colonies show no hemolytic reaction on blood agar. The culture is muddy in broth culture and sticks to the flask wall.
3.1.1.3. Actinomyces odontolyticus
A. odontolyticus is a gram-positive irregular bacillus (Figure 3.3(A) and (B)). It cannot grow in an anaerobic environment unless CO2 is added. Growth can be inhibited by 5–20% bile or 0.005% crystal violet. Characteristic colonies are shown in Figure 3.3(C)–(E). The DNA GC content is 62% when analyzed by Tm method. The type strain is ATCC17929 (NCTC9935, WVU867, CDCX363).
Dental plaque and dental calculus are the main habitats. This species is often involved in eye infections and is occasionally seen in advanced actinomycosis. The relationship between A. odontolyticus and periodontosis and dental caries remains to be confirmed.
A. odontolyticus can generate filiformed microcolonies. The mature colonies on the surface of BHI agar measures are less than or equal to 2 mm in diameter. They are white or gray-white, opaque, and do not show a central sag or filamentous edge. The important identifying feature of this species is that the colony turns dark red after 2 days of culture on the surface of blood agar under an anaerobic environment. The color clears after the cells are placed at room temperature.
3.1.1.4. Actinomyces viscosus
A. viscosus is a gram-positive irregular bacillus (Figure 3.4(A)–(C)). Primary cultures grow under anaerobic conditions and CO2 can stimulate its growth. Subcultures can grow in common atmospheric conditions. Some cells can grow at temperatures up to 45 °C. Characteristic colonies are shown in Figure 3.4(D) and (E). The DNA GC content ranges from 59% to 70% using the Tm method. The type strain is ATCC15987 (WVU745, CDCX603, A828).
Humans’ and other warm-blooded animals’ oral cavity, including subgingival plaque, transparent plaque, and dental calculus, are the main sites of growth. A. viscosus is one of the cariogenic bacteria found in root caries and is related to periapical infections, dacryosolenitis, and abdominal and faciocervical actinomycosis.
A. viscosus can generate filiformed microcolony. The mature colony does not have center sag. The typical colony is big and sticky.
3.1.2. Bifidobacterium
Bifidobacterium is a genus of bacteria with various forms; nonmotile they are gram-positive, nonsporulating, anaerobic bacilli. These bacteria were first isolated from infant feces and attracted attention because of their important physiological significance to the host organism. Species that are important human gut bacteria include Bifidobacterium bifidum, Bifidobacterium infantis, Bifidobacterium adolescentis, and Bifidobacterium longum. Bacteria isolated from the oral cavity belonging to the Bifidobacterium spp. include mainly Bifidobacterium dentium, Bifidobacterium breve, Bifidobacterium inopinatum, and Bifidobacterium denticolenu. The percent GC in Bifidobacterium DNA ranges from 55% to 67% when analyzed by the Tm or Bd method. The type species is B. bifidum.
The bacterial cells are short and thin, with pointed ends, and are irregular. They also appear as long cells with many branches and slightly branching spoon-shaped cells. Cells are arranged as single cells, chains, polymer-shaped, V-shaped, or palisade-shaped. Their distinct cell morphology can be helpful in differentiating bacteria belonging to this genus. For example, B. bifidum appear as flask-shaped cells, while Bifidobacterium asteroides are star-shaped. All members of this genus are gram-positive.
Bifidobacterium are anaerobes, and most strains cannot grow under 90% air and 10% CO2. Colonies formed on agar plates are convex, creamy or white, glossy, smooth, neat-edged, sticky, and soft.
The main terminal acid products in liquid culture medium containing glucose are acetic acid and lactic acid, but a few species also make formic acid and succinic acid. However, no butyric acid or propionic acid is formed, and no CO2 is generated (with the exception of gluconate degradation). Bifidobacterium can ferment carbohydrates to produce acid, and generally do not reduce nitrate or produce urease. They test positive using the catalase test.
3.1.2.1. Bifidobacterium dentium
Originally, B. dentium was isolated from pus specimens and named B. appendicitis. Later, researchers isolated similar bacteria from the adult dental caries, feces, and vagina, and they were then named Actinomyces eriksonii or grouped into B. adolescentis. According to later research, these bacteria make up an independent branch on the phylogenetic tree, and the species was named B. dentium in the 1970s. The percent GC in their DNA is 61% (Tm method) and type species is ATCC27534 (Reference strains B764).
The cells are anaerobic gram-positive irregular bacilli (Figure 3.5(A)). Some strains are resistant to oxygen in the presence of CO2. The optimal temperature for the growth of this bacterium is 37–41 °C, and the optimal pH value ranges from 6.5 to 7.0. TPY culture medium supplemented with neomycin, kanamycin, and various salt solutions is commonly used as the selective culture medium. Characteristic colonies are shown in Figure 3.5(B)–(D).
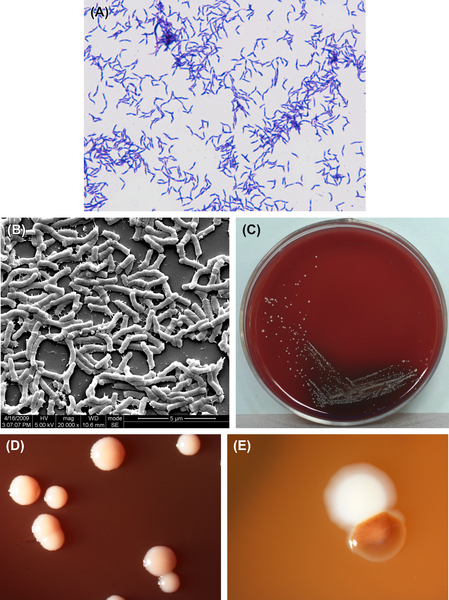
Figure 3.3 (A) Actinomyces odontolyticus cells (Gram stain). (B) A. odontolyticus cells (SEM). A. odontolyticus cells are mainly irregularly rod-shaped. Ball-like rods, branched rods, or filiform cells can also be observed. The cells are gram-positive. (C) A. odontolyticus colonies (BHI blood agar). (D) Red colonies of A. odontolyticus (stereomicroscope). (E) A. odontolyticus colonies in dental plaque (stereomicroscope).
B. dentium is biochemically active. It can ferment D-ribose, L-arabinose, lactose, sucrose, cellobiose, trehalose, raffinose, melibiose, mannitol, salicin, starch, galactose, maltose, fructose, xylose, mannose, and glucose to produce acid, but cannot ferment sorbitol and inulin. It cannot reduce nitrate and tests negative for both the urease test and the catalase test.
The distribution of the bacteria in the oral cavity and its pathogenic mechanism are not clearly characterized.
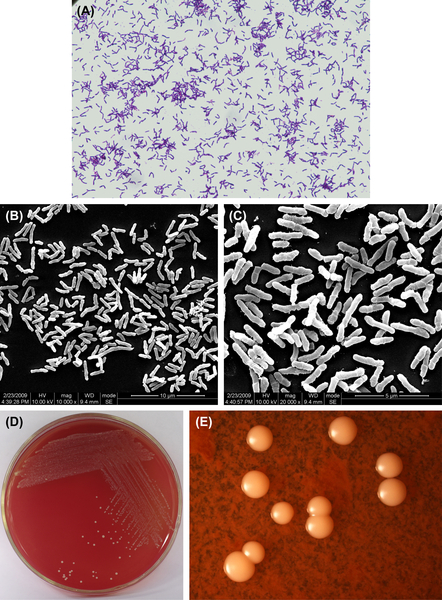
Figure 3.4 (A) Actinomyces viscosus cells (Gram stain). (B) A. viscosus cells (SEM). (C) A. viscosus cells (SEM). A. viscosus cells are mainly irregular short or moderate-length rod-shaped without spores. Branch rods or short filiform also can be seen, Gram stain is positive. (D) A. viscosus colonies (BHI blood agar). (E) A. viscosus colonies (stereomicroscope).
B. dentium forms colonies that can be described as spherical, lustrous, smooth, convex, gray-white, sticky and soft when plated on BHI blood agar and Bifidobacterium selective culture medium.
3.1.2.2. Bifidobacterium breve
B. breve is an anaerobic gram-positive irregular bacillus (Figure 3.6(A)–(C)). Its culture characteristics are similar to those of B. dentium, and characteristic colonies are shown in Figure 3.6(D) and (E). The percent GC in B. breve DNA is 58% by the Tm method and the type species is ATCC15700.
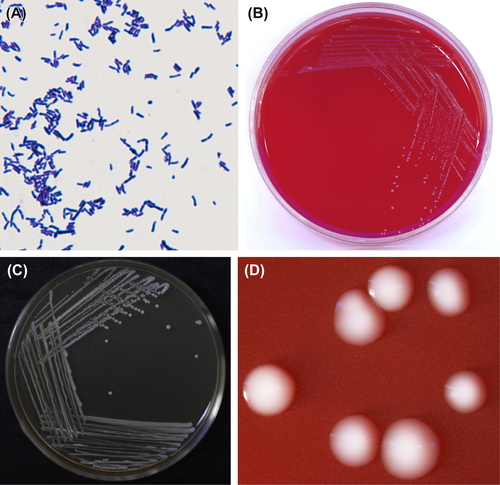
Figure 3.5 (A) Bifidobacterium dentium cells (Gram stain). (B) B. dentium colonies (BHI blood agar). (C) B. dentium colonies (B. dentium selective agar). (D) B. dentium colonies (stereomicroscope).
B. breve can ferment D-ribose, lactose and raffinose but does not ferment L-arabinose and starch.
B. breve on agar plates form colonies that are spherical, smooth, translucent, gray-white, sticky, and soft.
3.1.3. Lactobacillus
Lactobacillus is a group of anaerobic or microaerobic gram-positive bacilli that do not produce spores. Bacteria of this genus form part of the normal flora of the human oral cavity and intestinal tract. This genus is cariogenic, as they are detected in decayed oral cavity materials. This genus includes 44 species according to Bergey’s Manual of Systematic Bacteriology and also contains seven subspecies. The common species found in the oral cavity include Lactobacillus acidophilus, Lactobacillus salivavius, Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus brevis, and Lactobacillus casei. The percentage GC in the DNA is 40% (by either Tm method or Bd method). The type species is German type Lactobacillus.
The shapes and sizes of the bacterial cells can vary greatly. They can be vimineous, stubbed, bent, bacilliform, clavate, club-shaped, etc. However, most Lactobacillus cells are fairly regular with no branching. The cells are square or obtuse at the ends when compared with other gram-positive nonsporulating bacilli. They produce no spores and no capsules and stain gram-positive.
Surface culture on a solid medium is best when performed in anaerobic or microaerophilic conditions. However, some species must be cultivated under anaerobic conditions. Some members of this genus can grow within the 15–45 °C range, in the presence of 5–10% CO2, which promotes bacterial growth. As acid-producing bacteria, low pH Rogosa agar is the culture medium of choice for many strains of Lactobacillus. The optimal pH for growth is 5.5–6.2.
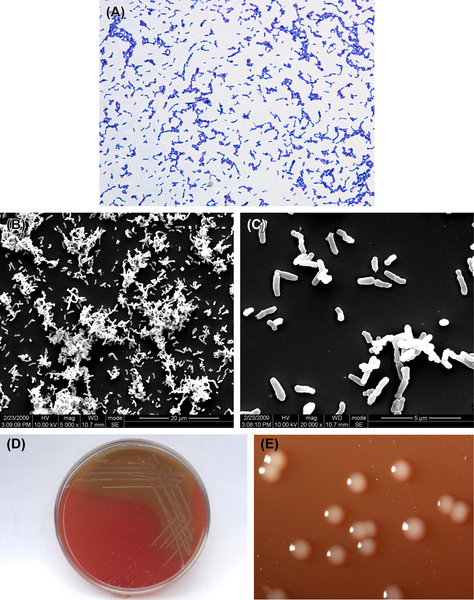
Figure 3.6 (A) B. breve cells (Gram stain). (B) B. breve cells (SEM). (C) B. breve cells (SEM) B. breve cells are thin and short bacilli. Nonsporulated, nonmotile, gram-positive. (D) B. breve colonies (BHI blood agar). (E) B. breve colonies (stereomicroscope).
The colonies are round, white or gray, transparent or nontransparent with a diameter from pinprick-sized to 2 mm on the agar surface. Smooth colonies are soft, raised, and lustrous, and the edge of the colony is neat. The surface of rough colonies is dry, flat, and lackluster, and the edge is not neat. The bacteria normally do not produce pigment.
Lactobacilli can ferment glucose to produce acid, and are negative for catalase, urease, and cytochrome enzyme. They do not produce benzpyrole, cannot reduce nitrate, cannot hydrolyze gelatin, and cannot produce H2S. Sugar fermentation test and arginine hydrolysis test can help identify the genus of bacteria.
The bacteria can promote the development of tooth decay, as its detection is significantly increased in deep caries material.
3.1.3.1. Lactobacillus acidophilus
These are gram-positive regularly shaped bacteria (Figure 3.7(A) and (B)) that grow well under anaerobic conditions. Cells grow well at 45 °C but do not grow at 15 °C. BHI blood agar and Rogosa agar are the commonly used media, and the latter is a selective medium. Characteristic colonies are shown in Figure 3.7(C)–(G).
L. acidophilus is obligate homofermentative bacteria. They can produce D– or L-lactic acid, ferment glucose, and most strains can also ferment starch. L. acidophilus cannot produce ammonia from arginine. The GC content in its genomic DNA is 32–37% (by Tm method or Bd method). The type strain is ATCC4356.
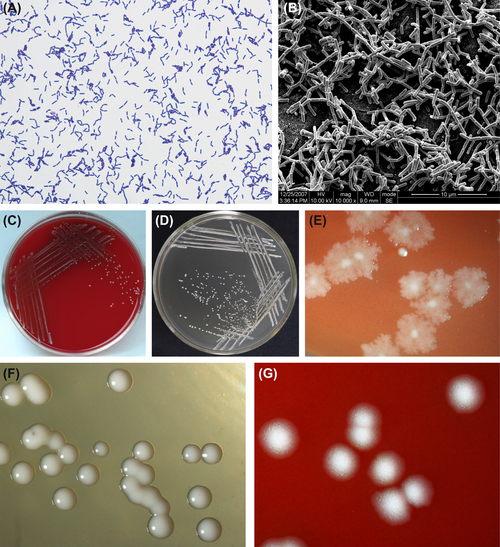
Figure 3.7 (A) Lactobacillus acidophilus cell (Gram stain). (B) L. acidophilus cell (SEM). Cells of L. acidophilus measure 0.3–0.4 μm × 1.5–4.0 μm in size. They are arranged as single cells, in pairs, or as short chains. They have rounded ends and no muramic acid in the cell wall. Cells stain gram-positive. (C) Colonies of L. acidophilus (BHI blood agar). (D) Colonies of L. acidophilus (Rogosa agar). (E) Rough colonies of L. acidophilus (stereomicroscope). (F) Smooth colonies of L. acidophilus
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


