Fig. 1.1
Human permanent molar crown section: the enamel is thick up to 2–3 mm over the tips of cusps and about 1–1.3 mm thick over the lateral surfaces (E enamel, D dentin, C cementum)
In young people the enamel is whiter and more yellowish in the elderly. In absence of the underlying dentin, as the incisal margin of anterior teeth, the color is more bluish. In cases of teeth with mineralization defects, the enamel color looks white-yellow, opaque, and brown-yellow and porous (see Chap. 6).
Enamel surface with time undergoes discoloration due to the greater presence of dyes and trace of minerals in the dentin and also due to the gradual increase of fluorapatite presence. Sometimes when the pulp has died, the dentin becomes discolored and darker. It may also become gray and brown in individuals who have assumed tetracycline antibiotics during dentin formative stages (development stages); consequently also the enamel appears to acquire the same coloration.
1.2.3 Ultrastructure of the Enamel
The ultrastructure of the enamel is formed of conglomerate of many units of enamel prisms (from 4 to 13 million depending on the volume of the tooth).
A prism has a cylindrical shape and it is in intimate contact one to another; they are arranged in overlapping wavy rows starting from the enamel–cement junction up to the cusps (Fig. 1.2). In cross section the prism has a typical key-hole aspect, the so-called head and tail; the disposition of the head of the prism between the two tails of the neighbor prisms leaves a very small empty space that is filled by organic substances. Each prism is covered by crystals and interprismatic substance with longitudinal arrangement called the sheath of the prism (Figs. 1.3 and 1.4).
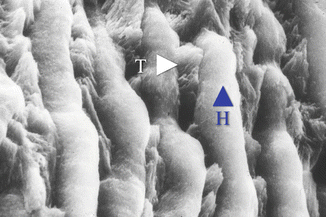
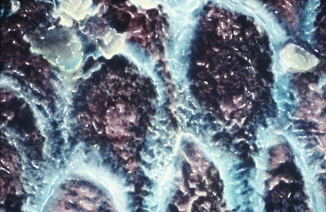


Fig. 1.2
SEM higher magnification image shows group of prism units in intimate contact one to one another. Underlying conglomerates of prisms (corresponding in section to the tail of prism, T) are emerging through the prisms (corresponding in section to the head of prism, H)

Fig. 1.3
SEM hand-painted image (5200×) of cross section of enamel prisms shows the typical key-hole aspect or so-called head and tail of the prism. The head of each prism is located between two tails of the neighbor prisms and leaves a very small empty space that is filled by organic substances (interprismatic space). Colored in blue is the sheath of the prism (Reprinted with permission from Fonzi et al. [4])

Fig. 1.4
SEM image at 1500× magnification shows the typical aspect of non-etched lateral surface enamel
Etching with common acids in adhesive procedures modify the enamel surface, resulting in the dissolution of the prism’s structure; depending on the part of surface etched, occlusal cusps or lateral (buccal and lingual), the central part of the crystal or its periphery is demineralized and so highlights the prism’s structure in a different way (Silverstone type I and II patterns) (Figs. 1.5, 1.6, 1.7, 1.8, and 1.9).
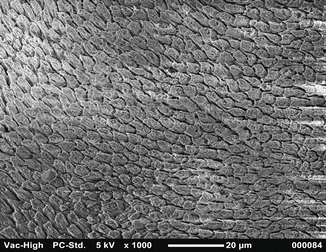





Fig. 1.5
SEM image at 1000× magnification shows buccal and lingual enamel surface etched with 37 % orthophosphoric acid: Silverstone type II pattern

Fig. 1.6
SEM image at 4000× magnification shows buccal and lingual enamel surface etched with 37 % orthophosphoric acid: Silverstone type II pattern

Fig. 1.7
SEM image at higher magnification (8000×) shows buccal and lingual enamel surface etched with 37 % orthophosphoric acid: Silverstone type II pattern. The etching works more on the periphery of the prism than on the core

Fig. 1.8
SEM image (1000×) of cross section of enamel prisms etched with 37 % orthophosphoric acid: Silverstone type I etching pattern. The acid works more on the core of the prism, typically on the occlusal enamel surface

Fig. 1.9
SEM image of cross section of enamel prisms etched with 37 % orthophosphoric acid: Silverstone type I etching pattern. The acid works more on the core of the prism, typically on the occlusal enamel surface
The enamel of marginal ridges of young permanent teeth and of the surface of primary teeth presents an irregular arrangement of the crystals, parallel to each other and perpendicular to the surface (aprismatic enamel), that confers a higher resistance to acid dissolution to the enamel (Fig. 1.10).


Fig. 1.10
SEM image (1000×) of aprismatic enamel also showing a development defect (lacuna)
The enamel–dentin junction is the border area between the enamel and the dentin. At microscopic observation it shows a wavy line with a more or less pronounced concavity facing the enamel. This arrangement favors the most intimate union of the dentin and the enamel. In correspondence of the cusps, the junction is crossed by numerous terminal branches of the dental tubules [1] (Figs. 1.11 and 1.12).
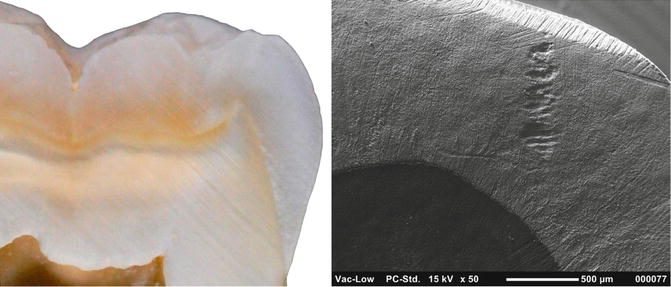
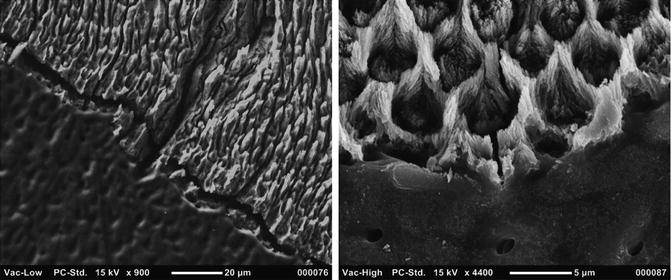

Fig. 1.11
Microphotography and SEM image (50×) of enamel–dentin junction

Fig. 1.12
SEM observation off enamel–dentin junction: left lower magnification (900×) shows a wavy lines with a concavity facing the enamel that favors a most intimate union of tissues; right higher magnification (4400×) shows dentin tubules and enamel prisms just on the border
Enamel modification are observable on the surface of the enamel; the so-called perichimita or incremental lines are layers of prisms spaced every 20–80 μm overlapping from the enamel–cement junction to the internal layers of the enamel that are formed by the eruption onwards [1] (Fig. 1.13).
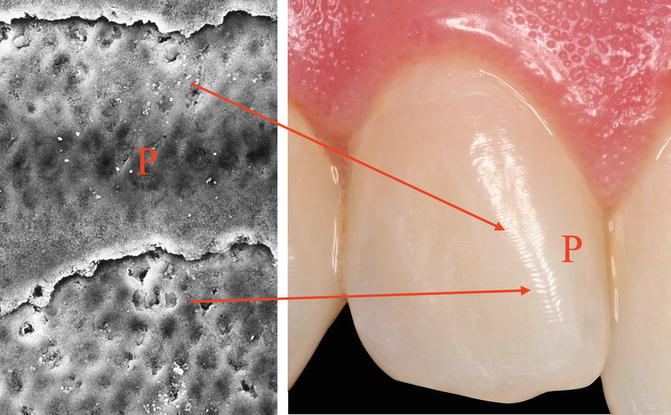

Fig. 1.13
Microphotography and SEM image of perichimita (P) or incremental lines. The layers of prisms are spaced 20–80 μm
Sometimes a young enamel surface shows development anomalies (qualitative and quantitative) in the form of holes or gaps or hypomineralization that facilitate the start of the carious process.
1.3 Pulp–Dentin Complex
Pulp–dentin complex is a specialized connective tissue of the tooth. Both the pulp and dentin originate from the mesodermal tissue derived from the dental papilla. The dentin is a highly mineralized and avascular tissue; on the opposite, dental pulp is a highly vascularized tissue, with rich innervation. The dentin forms the largest part of the tooth, being the bearing structure of almost the entire length of the tooth [1]. The dentin surrounds the pulp tissue, from the pulp chamber up to the radicular canal. Externally it is covered by enamel on the crown and by cementum on the root/s of the tooth. The initial stages of odontogenesis begin in the external area of the dentin and continue with a centripetal direction; effectively, the odontoblasts move towards the inside of the tooth, leaving during their path their cytoplasmatic extension, called the odontoblast process, included within the secreted organic matrix of the primary dentin. The dentin consists mostly of an irregular weave of collagen fibrils intimately interwoven with hydroxyapatite crystals. The dentin progressively mineralized due to the deposition of hydroxyapatite crystals to form the dentin tubules that represent the basic architecture of the dentin. Each odontoblastic process is located in one dentinal tubule and its cellular body do not remain included in the produced tissue, but in the outer layer of the pulp, and this is why these two tissues are closely related to one another and are so named pulp–dentin complex [1] (Fig. 1.14). The inner layer of the dentin, the predentin, is not mineralized. The odontoblasts make the formation of the new dentin layer, just above the pulp, possible; in fact the dentin undergoes several changes in its volume during the life, due to its continuous formation from the periphery of the underlying pulp tissue (secondary dentin) or to formation of a barrier as a reaction from irritating external stimuli (tertiary dentin: reactive and sclerotic), with subsequent reduction of the pulp chamber volume [1].
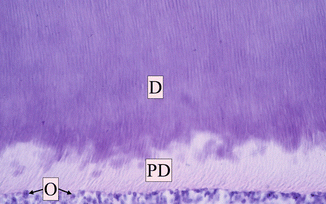

Fig. 1.14
Dentin pulp complex at optical microscopy (OM). Dentin (D) and predentin (PD) with dentin tubules; the odontoblasts (O) are aligned on the external layer of the pulp (Reprinted with permission from Fonzi et al. [5])
1.4 Dentin
1.4.1 Chemical Properties of Dentin
The dentin is composed of 70 % inorganic material, 18 % organic matrix, and 12 % water in weight.
The inorganic material consists mostly of hydroxyapatite crystals, whose size is 50–60 nm in length and 20–30 nm in width; carbonate and traces of other elements (F, Na, Mg, C, Ba, Al, Z, K, Fe) are also present [3]. The fluoride concentration in the inorganic phases seems to be influenced by the content of fluorine ions in the diet and by the amount of time of tooth exposure to the same fluorides.
The organic matrix consists essentially of type I collagen and non-collagen proteins that also work as regulator of the mineralization of dentin (proteoglycans, phosphoproteins, glycoproteins, gamma-carboxyglutamic acid, etc.); there is lack of type III collagen fibers.
The type I collagen represents 80–90 % of the organic matrix. Proteoglycans (protein–glycosaminoglycan complexes) act as regulators of the fibrillogenesis during the dentinogenesis and, therefore, for the organization of the predentin matrix, with a specific role for the inhibition of the formation of the mineral phase.
Phosphoproteins (PP-H) play a role in the front of mineralization of the dentin, facilitating the formation of hydroxyapatite crystals for their ability to bind Ca and P; the gamma-carboxyglutamic acid has the same role to bind proteins (Gla protein).
Other organic components are lipids (cholesterol and phospholipids), citric acid, and lactates. According to their specific metabolic role, these different organic components are mainly present in the different areas of dentin.
1.4.2 Physical Properties of Dentin
The dentin is less mineralized and more elastic than enamel (Ca/P ratio lower: 1.96 ± 0.062), but it is stiffer and more mineralized than the cement and the bone. Dentin has a pale yellow color, thus affecting the color of the crown. It is very permeable for the presence of the tubular system that crosses it for the whole thickness, from the pulp to the enamel-dentin and dentin–cement junction. The presence of dentin tubules also affects conductivity and thermal diffusivity of the dentin; however, because of its compact shape, the presence of dentin tubules does not allow the passage of liquids and microorganisms. The tubular structure and organic collagen matrix give the dentin a high degree of elasticity.
1.4.3 Ultrastucture of the Dentin
A chronological and functional subdivision of the dentin considers primary, secondary, and tertiary dentin. The dentin can be divided, from the border with the pulp to the junction with the enamel, into three regions, each one with specific characteristics: the predentin, the circumpulpal dentin, and the mantle dentin (Fig. 1.15).
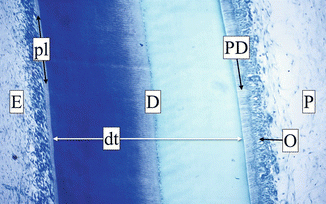

Fig. 1.15
Cross section of a tooth crown at optical microscopy (OM). From left to right are the visible enamel (E); the proteic layer (pl); the dentin (D), crossed by dentin tubules (dt); the non-mineralized predentin (PD); and the odontoblast (O) on the external part of pulp tissue (P)
1.4.3.1 Predentin
The predentin is a non-mineralized layer (15–40 μm thick) of the dentin, placed just above the pulp and below the circumpulpal dentin. The predentin is continuously formed and persists throughout the life of the tooth; its formation is followed by the simultaneous mineralization of the dentin at the same speed with which it is formed, so that the thickness of the predentin remains constant. The type I collagen fibrils are arranged parallel to the boundary line between the pulp and dentin, to form an overlapping network intertwined in a messy way. During the early stages of dentin mineralization, at the border with circumpulpal dentin, crystals of hydroxyapatite are deposited inside and around the collagen fibers of the predentin, in the form of spherical clusters growing in centrifugal direction. These structures are called calcospherites and represent the mineralization front of the dentin (Fig. 1.16). Getting closer to the front of mineralization of the predentin, the collagen network becomes more and more compact, as the result of a process of maturation of interfibrillary bonds. Therefore, predentin can be considered as an area of maturation of collagen fibers and of inhibition of mineralization.
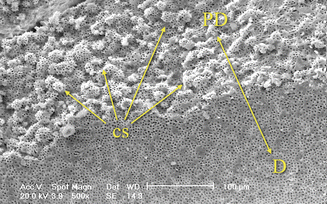

Fig. 1.16
Border between predentin and circumpulpal dentin presents globular structures called calcospherites and represents the mineralization front of the dentin
1.4.3.2 Circumpulpal Dentin
It is the main and more calcified mass of dentin, located between the predentin and the mantle dentin. The type I collagen fibrils are arranged perpendicularly to the dentinal tubules; they increase in number and diameter towards the junction with the enamel, becoming more compact. Some thin areas of circumpulpal dentin present hypomineralized and not mineralized matrix; these areas are formed as the result of the lacking coalescence of the calcospherites and appear as irregular or roughly spherical and apparently empty spaces, called interglobular dentin.
1.4.3.3 Mantle Dentin
It is the thin peripheral layer of dentin (thickness of about 15–20 μm) placed just below the enamel-dentin junction and above the circumpulpal dentin. It is the first dentin to be formed and differs from the circumpulpal dentin for a different arrangement and a greater diameter of the collagen fibrils (0.1–0.2 μm). The collagen fibrils are perpendicularly aligned to the enamel-dentin junction and are covered by glycosaminoglycans. Aperiodic fibrils (type III collagen) are present among the collagen fibrils. The mantle dentin is less mineralized of the circumpulpal dentin.
1.4.3.4 Dentinal Tubules
Dentinal tubules present different size and distribution in different areas of the tooth. Their diameter is about 2–3 μm in proximity of the pulp, and it is reduced to 0.5–0.9 μm in proximity to the junction with the enamel (Fig. 1.17). Also their number varies from about 45,000 per mm2 in the proximity of the pulp to 29,000–35,000 per mm2 in the intermediate part, up to about 20,000 per mm2 close to the enamel. The reduction in diameter is due to an increase of the peritubular dentin, while the decrease in the number of tubules is only apparent, because of a gradual increase of the surface area of the tooth towards the enamel–dentin junction. In fact, also considering the decrease in tubular diameter, the distance between two adjacent tubules is 15 μm in the outer periphery of the dentin and only 6 μm in proximity of the pulp (Fig. 1.18
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


