Extrinsic
Chromogenic bacteria, food and drink, smoking, medicine, plaque, tartar, products with chlorhexidine, metal salt
Intrinsic preeruptive
Fluorosis, tetracycline, traumas, imperfect amelogenesis
Intrinsic posteruptive
Inadequate dental treatments, trauma, tertiary dentin
Dyschromia can be isolated, that is to say localized in one or more elements, or generalized, meaning extended to most of the elements.
The classification of dyschromia is essential to evaluate the predictability of bleaching and the stability of the result.
While extrinsic dyschromia can be easily treated at home by the patient with toothpaste or by professional polishing techniques, air-flow polishing, the intrinsic dyschromia requires professional bleaching treatments or esthetic dentistry treatments (composite resins, veneers, crowns).
9.2 Extrinsic Dyschromia
Extrinsic dyschromia is caused by external compounds incorporated in the acquired film on the enamel surface, which cause coloration as a result of the chemical interaction between pigmenting substances and the surface of the tooth [11]. Among these extrinsic factors there are chromogenic bacteria (actinomyces), food and drink, smoking, drugs, plaque, tartar, products with chlorhexidine, metal salt, etc.
The clinical classification by Nathoo S.A. is the most frequently used and is based on clinical pigmentation that distinguishes between three kinds of discoloration [12].
9.2.1 Direct Tooth Discoloration Type N1
Colored compounds (chromogens) links to the dental surface provoking pigmentation; the color of the pigmentation is similar to that of the chromogen. Colored food and drinks (carrots, turnips, cherries, liquorice, coffee, tea, wine) directly depose chromogenic substances; the tannins contained in the drinks mentioned interact through mechanisms of ionic exchange.
Metallic ions are also one of the causes of these kinds of discoloration (green pigmentation from copper or black from iron) (Figs. 9.1 and 9.2).
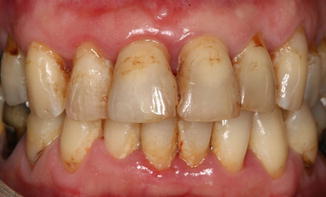
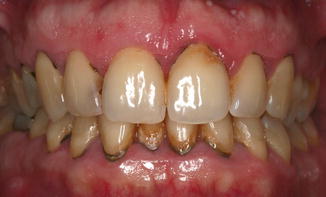

Fig. 9.1
Concomitant extrinsic and intrinsic posteruptive discoloration of the teeth. The extraoral image shows the presence of plaque; tartar, associated to discoloration due to infiltrated composite fillings (on teeth #1.1, #2.3, #3.4, #4.4, #4.6); decay (on teeth #1.4, #2.1, #2.2); several decalcification; and abrasion with tertiary dentin on tooth #2–4)

Fig. 9.2
Concomitant extrinsic and intrinsic posteruptive discoloration of the teeth. The extraoral image shows decay on tooth #1.2, dyschromia of teeth #1.4, 2.4 and 4.6 for pulp necrosis. Decalcification of tooth #2.1 and deposits of tartar and plaque on the neck surface of the teeth
9.2.2 Direct Tooth Discoloration Type N2
Colored compounds change color after linking to the tooth. They are usually present in the interproximal or cervical areas of the aged teeth. This could be caused by modification induced on the proteins of the acquired film.
9.2.3 Direct Tooth Discoloration Type N3
The pre-chromogen is a colorless compound, which, through different chemo-physical reactions, after linking to the enamel leads to chemical reactions which produce pigmentation. The discolorations caused by chlorhexidine, stannous fluoride are included in this group Figs. 9.3, 9.4, and 9.5.
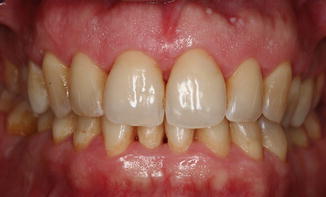
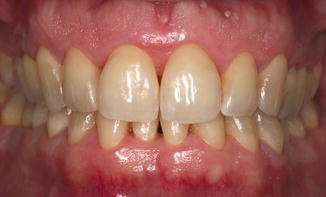
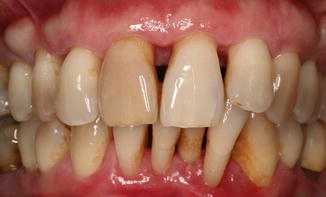

Fig. 9.3
The extraoral image at 1 year follow-up after scaling and polishing and restorations. Note the Nathoo type N3 dyschromia due to excessive use of chlorhexidine rinse (orange staining)

Fig. 9.4
The extraoral image at 1.5 year follow-up shows better hygiene due to the improved compliance of the patient

Fig. 9.5
Concomitant extrinsic and intrinsic posteruptive discoloration of the teeth. The extrinsic staining is due to continuous use of chlorhexidine rinse (orange staining). The intrinsic posteruptive dyschromia (type N3) of the upper right incisor is due to a reactive tertiary dentin consequent to a previous trauma; the upper right incisor is vital
9.3 Intrinsic Dyschromia
They are provoked by different factors that modify the coloration of the tooth linking to the organic and mineral structure of the tooth, during both the development and mineralization period.
They involve the entire tooth and are divided into:
(a)
Preeruptive dyschromia: fluorosis, tetracycline, trauma, and amelogenesis imperfecta
(b)
Posteruptive dyschromia: inadequate dental treatments, trauma, and tertiary dentin
The most common among preeruptive intrinsic discolorations is fluorosis; it is due to a fluoride overexposure, usually for its high concentration in the water of some geographical areas. Its accumulation is extremely harmful not only for teeth, but also for the liver and for bones. Fluorosis was classified by Dean (1924, 1942) as questionable, very mild, mild, moderate, and severe, and it is important to be able to recognize its seriousness, depending on the intensity of discoloration, because it can represent a strong limit to conventional bleaching systems [13, 14].
Tetracycline discoloration can be preeruptive, when it is caused by consumption of this antibiotic during the second or third months of pregnancy, or posteruptive during the first years (6–8 years), and the entity and quality of the alteration (grey brown/yellow) depends on the ingested dose [1]. These pigmentations are the most difficult to treat (Figs. 9.6 and 9.7).
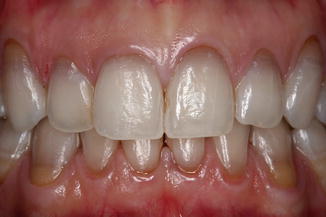
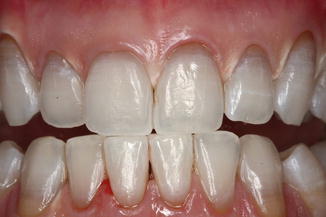

Fig. 9.6
A 40-year-old female that took tetracycline medication during childhood. The extraoral image shows the typical intrinsic posteruptive dyschromia (grey brown/yellow) more evident to the cervical side of the teeth due to the thinner width of enamel

Fig. 9.7
The immediate post-bleaching image shows presence of little damage to the gingiva and good bleaching result, more evident on the crown than on the neck. The bleaching has been performed with 532 nm KTP laser
Amelogenesis imperfecta (malformation of the enamel) can be caused by:
-
Hematological disorders (hemolytic disease of the newborn, thalassemia, anemia falciparum)
-
Malnutrition, lack of vitamin D, and native hypocalcemia
-
Maternal hypo-/hyperthyroidism and maternal diabetes
-
Mental delay, cerebral paralysis, and cardiac malformation
-
Ectodermal dysplasia
Intrinsic posteruptive discoloration can also depend on other factors:
-
Decalcification
-
Carious lesions
-
Childhood diseases (e.g., measles)
-
Endodontic treatments
-
Trauma and pulp necrosis (red blood cell hemolysis)
Dental trauma can provoke abnormal discoloration through different pathogenetic mechanisms [1]:
-
Reactive dentin: The reaction of the pulp-dental organ, after a traumatic damaging event, can provoke a conspicuous deposition of reaction dentin to protect the pulp which usually remains vital. The increase in dentin thickness provokes a loss of transparency of the tooth with relative decoloration (see Sect. 1.4.4.3 and Fig. 9.5).
-
Pulp hemorrhage and pulp necrosis: In the case of pulp hyperaemia and/or pulpitis, post-bleeding hemolysis of red blood cells provokes the liberation of hematoidin and hemosiderin, which, combining with sulfuric anhydride derived from the decomposition of pulp proteins, creates iron sulfide. This substance penetrates the dental tubules, and it is responsible for their dark color (Figs. 9.8, 9.9, and 9.10).
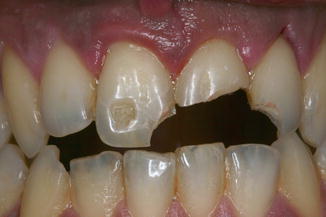 Fig. 9.8A 28-year-old male the day after an accident; trauma involved the two central incisors with complicated and not complicated enamel-dentin fractures as well as a not complicated fracture on left lateral incisor. Also many enamel cracks and infraction are visible
Fig. 9.8A 28-year-old male the day after an accident; trauma involved the two central incisors with complicated and not complicated enamel-dentin fractures as well as a not complicated fracture on left lateral incisor. Also many enamel cracks and infraction are visible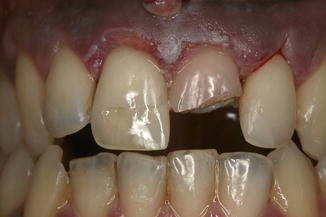 Fig. 9.9The image 1 week post laser pulpotomy and MTA capping shows the characteristic pink coloration after an internal hemorrhage with hemolysis of red blood: the tooth remained vital
Fig. 9.9The image 1 week post laser pulpotomy and MTA capping shows the characteristic pink coloration after an internal hemorrhage with hemolysis of red blood: the tooth remained vital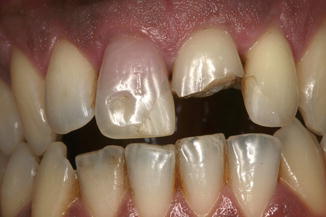 Fig. 9.10The image 2 weeks later shows the spontaneous resolution of the internal hemorrhage on the left central incisor and dyschromia and internal hemorrhage on the right central incisor due to irreversible pulpitis
Fig. 9.10The image 2 weeks later shows the spontaneous resolution of the internal hemorrhage on the left central incisor and dyschromia and internal hemorrhage on the right central incisor due to irreversible pulpitis
In case of necrosis of the pulp, the final products of decomposition (hydrogen sulfide, carbon dioxide, fatty acids, etc.) can also penetrate the dental tubules and cause abnormal pigmentation [15]. If coloration and pathology are irreversible, endodontic treatment is necessary and the sodium hypochlorite irrigation allows cleaning the dental tubules from the products of degradation and from red blood cells. A non-vital bleaching procedure will follow, if needed.
9.4 Dental Bleaching and Bleaching Agents
Dental bleaching causes the breakage of chromophore groups present in the organic and inorganic components of the tooth due to a redox chemical reaction. Oxygen radicals are liberated by the peroxides contained into the bleaching products and diffused through the tooth’s hard tissue up to the amelo-dentin (enamel-dentin) junction. This specific redox chemical process degrades the color systems (quinone and aromatic systems) destroying the double links and producing composites with a low molecular weight, mainly carboxylic, colorless, and water-soluble acids, easily removable by washing.
The main bleaching agents in dentistry are the hydrogen peroxide (H2O2) and carbamide peroxide, which, in the presence of a catalyst, splits into hydrogen peroxide and urea [16–22], but oxygen radicals are those which determine the whitening process. The chemical reaction, in the presence of activators and photo-activators, can be accelerated through the application of heat and/or by light (both ordinary or laser light) which are able to accelerate and to increase the intensity of the chemical reaction, helping the dissociation of the peroxide and increasing the formation of oxygen and ion peroxide [23] (Figs. 9.11, 9.12, 9.13, and 9.14).





Fig. 9.11
Optical microscope polarized light observation of the enamel surface immediately after placing a gel containing 38 % hydrogen peroxide: no activation (time 0). The image shows the initial formation of oxygen microbubbles on the enamel surface (Courtesy of University of Genova, ©2012)

Fig. 9.12
Optical microscope polarized light observation of the enamel surface after placing a gel containing 38 % hydrogen peroxide: no activation. Note the increased formation of oxygen microbubbles after 30 min (Courtesy of University of Genova, ©2012)

Fig. 9.13
Optical microscope polarized light observation of the enamel surface immediately after laser activation of the gel containing 38 % hydrogen peroxide; 980 nm laser activation for 30s, no-contact handpiece. The initial formation of oxygen microbubbles on the enamel surface is more evident than in the case of self-activation (Courtesy of University of Genova, ©2012)

Fig. 9.14
Optical microscope polarized light observation of the enamel surface 30 min after laser activation of the gel containing 38 % hydrogen peroxide; 980 nm laser activation for 30s, no-contact handpiece. The formation of oxygen microbubbles on the enamel surface is much higher than in the case of self-activation (Courtesy of University of Genova, ©2012)
Nowadays the techniques used involve the use of hydrogen peroxide (3–38 %) and carbamide peroxide (10–40 %), both auto- or photo-activated, or a mix of sodium perborate and hydrogen peroxide. These products can be used with different application times and different concentration, home bleaching, in-office bleaching, or power bleaching (Fig. 9.15).
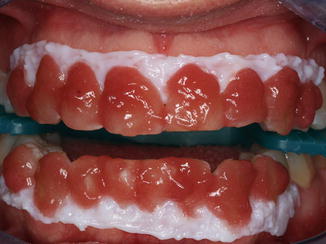

Fig. 9.15
Opalescence Boost PF (Ultradent, UT, USA) is a 40 % hydrogen peroxide chemically activated power whitening gel that doesn’t require heating or lighting to work. Time suggested by the manufacturer is 40 min in the dental chair. The gel contains PF (potassium nitrate and fluoride) that has been shown to help reduce sensitivity
The bleaching technique increases dentin permeability and can also intensify dental sensitivity, especially with longer treatment and a raised temperature (see Sect. 9.5).
In Europe the scientific committee on consumer safety (CSSC) decided to limit the use of bleaching products or dental brightening products to that containing hydrogen peroxide in a range of 0.1 % up to 6 % (20 volumes), present (free) or liberated by other composites or mixes contained in those products; these products can be safely administered only by dentists, once the absence of risk factors is guaranteed. Use is permitted only for those who are 18 years old or over [24–26].
9.5 Collateral Unwanted Effects
The side effects caused by tooth whitening agents can be divided into major and minor.
Among the minor side effects of tooth whitening are:
-
Posttreatment dentin hypersensitivity, usually transitory.
-
Temporary reduction of the adhesion of composite resins, for which it is advisable to postpone subsequent restoration for at least a couple of weeks.
-
Corrosion of the superficial layer of the amalgam filling with possible release of silver ions and mercury, if the bleaching agent comes in contact with an amalgam filling.
-
Gingival irritation due to the infiltration and caustic action of the peroxide under the protective gingival barrier, which can be quickly resolved (2–3 days) and helped by the use of a vitamin E-based cream (Figs. 9.16, 9.17, and 9.18).
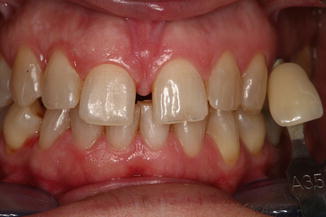 Fig. 9.16A 48-year-old female required an esthetic whitening treatment. The starting color was previously evaluated
Fig. 9.16A 48-year-old female required an esthetic whitening treatment. The starting color was previously evaluated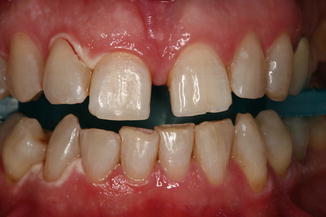 Fig. 9.17Immediately post-op image shows mild gingival irritation due to contact with the bleaching gel. Laser activation with 532 nm KTP laser, using a specific handpiece; total procedure time 12 min. A vitamin E-based cream was applied for 2 days on the gingiva to reduce the sensitivity and accelerate the healing
Fig. 9.17Immediately post-op image shows mild gingival irritation due to contact with the bleaching gel. Laser activation with 532 nm KTP laser, using a specific handpiece; total procedure time 12 min. A vitamin E-based cream was applied for 2 days on the gingiva to reduce the sensitivity and accelerate the healing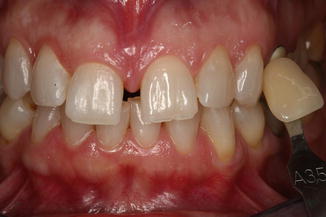 Fig. 9.18One week post-op image shows the healing of the gingival irritation and the very good esthetic result. The bleaching has been performed with 532 nm KTP laser
Fig. 9.18One week post-op image shows the healing of the gingival irritation and the very good esthetic result. The bleaching has been performed with 532 nm KTP laser -
Algic-dysfunctional TMJ syndrome because of long opening of the mouth during the bleaching session.
The major side effects are:
-
The possibility of systematic acute toxicity, only in the case of accidental ingestion of a large quantity of hydrogen peroxide. Ingestion can provoke abdominal cramps and also sensorial disorientation.
-
Possible chronic systematic toxicity has not been clinically proved, even with long use of mouthwashes containing peroxides or perborate (until 3 years).
-
Over-bleaching.
Tooth bleaching happens through the chemical process of breaking double carbon-carbon links. If the exposure time of the tooth to bleaching gel is longer more than 20 min, it is possible to overlap the optimal “whitening” to a pathologic condition called over-bleaching, which involves the formation of the final products of oxidation (H2O and CO2). At the structural and clinical level, the over-bleaching shows an increased porosity of the enamel, until arrival at the loss of hard substance, increase in dental sensitivity, and the need for devitalization of the tooth [27]. The procedure time must therefore be kept under 20 min (Figs. 9.19, 9.20, 9.21, and 9.22).
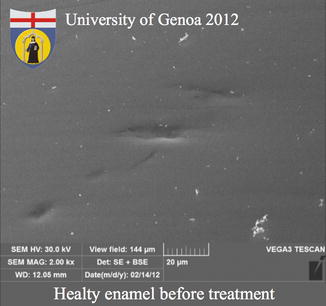


Fig. 9.19
Image of the healthy intact enamel before the application of the bleaching gel (Courtesy of University of Genova, ©2012)

Fig. 9.20
Image of enamel surface washed for 60 s after the application of a 38 % hydrogen peroxide gel, activated by a 980 nm laser for 30 s and remained on the surface for 24 min. Initial reversible alterations of the prismatic enamel structure are visible in the left-top side of the image. The alterations have a diameter of 0.5 μm (Courtesy of University of Genova, ©2012)
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses



