Fig. 4.1
Dental delivery system for nitrous oxide administration
Clinical
Nitrous oxide is an excellent tool for aiding children who are mildly to moderately anxious in the dental chair. Normally, the concentration of nitrous oxide for wary or mildly fearful pediatric patients ranges from 25 to 50 %. It is the author’s opinion that the 35–45 % range works best for most children. Moderately fearful children may require higher concentrations of nitrous oxide initially, but once benefitting from its effects, the concentration can be lowered and still maintains its effectiveness.
Like all tools for guiding patient’s behaviors and responsiveness, the effectiveness of nitrous oxide is greatly dependent on the talents of the dentist who must have good “bedside” manners, a calming disposition, in-depth working knowledge of behavioral guidance techniques, and confidence in his/her skills of behavior guidance. One of the first steps, other than gaining the patient’s trust, in the successful use of nitrous oxide is introducing the child to the hood in a way that maximizes his/her curiosity and acceptance and minimizes the child’s anxieties about personal intrusiveness or fears of suffocation.
Ideally the child will accept the nasal hood. If this is the case, the dentist can begin the most frequently used technique of administering nitrous oxide which is referred to as titration. Titration is the process of slowly increasing the concentration of nitrous oxide in small steps wherein until the child exhibits, characteristically, ideal clinical signs and symptoms. For example, the patient may be started with a concentration of 15 % nitrous oxide and oxygen and allowed to inhale this mixture for a period of a minute or two, then the concentration is increased by 5 or 10 %, and again the patient inhales this mixture for another minute or two. At each step or change in concentration, the dentist is observing the patient for positive signs such as staring ahead into space and body posturing of quietness, relaxation, and warm, opened hands as well as querying the patient about how they feel (Fig. 4.2) or possibly suggesting what they should be feeling. If the concentration becomes too high, the patient may become agitated, have more spontaneous movement, clench their jaws, and even panic and quickly pull the nasal hood off their nose. Usually the idea concentration of nitrous oxide for children is between 35 and 50 %. Higher levels can be used under certain circumstances as will be explained shortly.
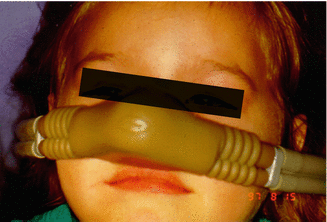

Fig. 4.2
Patient receiving nitrous oxide. Note slight smile and faraway stare as if “look at the stars”
Some children will not readily accept the hood or already be in such a state of agitation and disruptiveness that normal placement and titration of nitrous oxide is impossible. A second means of administering nitrous oxide is possibly now indicated. This technique is referred to as the rapid technique of administration of nitrous oxide. It should be noted that this is an aggressive technique usually involving the dentist and dental assistant but is surprisingly a success on many children. The rapid technique involves the dentist placing the hood and tubing between the thumb and first finger of each hand and with the palms of the hands placed on the sides of the child’s face, holding the nitrous oxide slightly off the face but covering the nose and mouth area of the face (Fig. 4.3). Remember the child will be struggling and want to move the head from side to side as well as turn away and/or raise their hands to grab the dentist’s hands and nasal hood. The dentist simply follows the head movements using his cupped palms which are gently touching the sides of the child’s face. During this phase, the dentist is constantly informing the child that everything will be OK. Simultaneously and in concert, the dental assistant now comes into play by leaning protectively over the top of the child’s body near his/her waistline and holding the child’s hands. Sometimes the parent, if present, is willing to do the same; alternatively, the child may be restrained in a passive device (e.g., Papoose board) if a decision is made to try this rapid-induction technique.
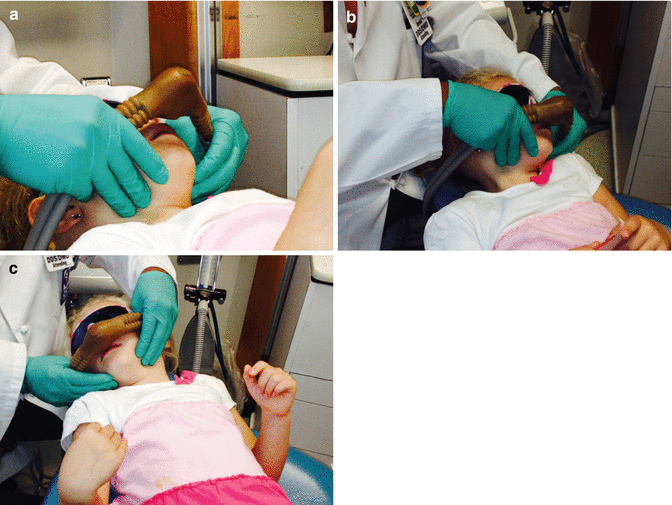

Fig. 4.3
Administration of high concentration of nitrous oxide to patient in rapid-induction technique.While the patient typically moves head from side to side (b and c), the dentist uses hands to stabilize the hood slightly off the face and over the nose and mouth of the patient (a)
It should be noted that the nitrous oxide concentration is turned to 70 % immediately before the beginning of the rapid-induction technique. If the child does not settle down within 6–10 min at this concentration, then the technique should be abandoned and another alternative treatment should be considered (e.g., general anesthesia). If the child does begin to calm down (and this doesn’t happen like the flip of a switch) and appears to be “listening” to the dentist, then the concentration should be turned down to 50 %. Talking with the child and giving positive verbal feedback is also helpful at this time. As mentioned previously, this technique surprisingly works frequently especially with preschoolers.
Although beyond the scope of the purposes of this writing, nitrous oxide hygiene should be briefly mentioned. For patient and possibly more for the dental team, good nitrous oxide hygiene should always be practiced. This includes minimizing nitrous oxide in the ambient air by using a well-designed scavenging system, use of fans, and rapid turnover of room air circulation. It should be noted that children who are too talkative or cry throughout the dental procedure, thus poorly benefitting from its use, tend to breathe or shunt more of the nitrous oxide into the ambient air of the operatory. Hence, for these children, the practitioner should probably terminate its delivery and use other techniques including rescheduling for a sedation appointment.
The nitrous delivery system should be checked daily for leaks in the ventilation bag as well as the tubing leading from the manifold to the patient. The bag should be changed on a regular basis as the gases tend to dry out the bag causing tiny cracks particularly along crease lines in the bag. Additionally, it is advisable to have periodic testing of the dental operatories by an independent service and make every effort to keep the concentration of nitrous oxide in the ambient air at 25 ppm or less. Compliance with state dental regulations related to nitrous oxide use in the office is also a must for every practitioner who uses nitrous oxide in their offices.
Benzodiazepines
We may know more about benzodiazepines than any other sedatives (e.g., chloral hydrate) despite their shorter clinical history. Benzodiazepines are sedative agents that characteristically have properties causing variable degrees of skeletal motor inhibition, sedation, hypnotic effects (sleep-inducing), anticonvulsant, anxiolytic, and some amnesia. The properties of the benzodiazepines are such that each benzodiazepine may possess some or all of the common effects usually attributable to this class of drugs but expressed in variable and pronounced ways allowing selections of benzodiazepines to address specified clinical needs.
Benzodiazepines are thought to act primarily in the CNS as agonists affecting a specific subtype of γ-aminobutyric acid receptor complex called GABAA. GABAA mediates postsynaptic inhibitory activity on other systems of the CNS through control of Cl− gates in neurons. GABA, a neurotransmitter, acts on Cl− gates in neurons causing an opening for Cl− ions to flow into the neuron hyperpolarizing it. Thus, GABA receptors which are actually large macromolecules capable of being activated by various substances are thought to have dominant inhibitory effects within the CNS. The other subtype of GABA receptor complex is a G-protein-linked receptor that apparently does not clinically respond to benzodiazepines. There are actually several benzodiazepine “receptors” within the GABA receptor complex mostly found in the CNS that may be responsible, to some degree, in causing the different benzodiazepine effects (e.g., sleep-inducing). However, this may be a simplistic view of the myriad of benzodiazepine profiles. Benzodiazepines may influence other neurotransmitter receptors other than GABA complexes (e.g., 5-hydroxytryptamine), and the interactive complexities may orchestrate in a way in producing clinically recognized effects.
Benzodiazepines are metabolized via the hepatic microsomal enzyme system and primarily excreted through the kidneys. The different benzodiazepines are metabolized differentially, and many of these agents are metabolized into other benzodiazepine derivatives which may also cause some minor clinical effects. Likewise, variations in lipid solubility, rates of protein binding, and distribution in fatty tissues account for differences in onset, duration of action, and elimination from the body. Care must be exercised to prevent possible adverse situations whenever benzodiazepines are administered in the presence of other agents or substances that interact with the hepatic microsomal enzyme system (e.g., grapefruit juice).
Clinical
By far, the most common benzodiazepines used for pediatric sedation is midazolam. It has a rapid onset and short duration of action, but contains no analgesic properties. Depending on the dose administered, typical behaviors can be observed after its oral administration. Typically, changes in patient behaviors can be perceived in less than 10 min. Generally, the child is less active and slightly more introverted or quiet. Relaxation of the limbs and even less mobility shortly becomes apparent. Eventually the child may become clinically limp and unable to stand or sit unaided, but still maintains eye contact. Respiration and cardiovascular parameters usually remain within appropriate clinical ranges for the patient’s age. Hiccups are occasionally witnessed and will disappear within 15–30 min in most cases. Any movements or verbalizations associated with initial procedural events are present, but blunted for a period of 15–25 min; however, after this time frame, in many cases impatience and intense reactivity to procedures become growing prominent.
Benzodiazepines can cause drowsiness, respiratory depression, allergies, and, probably most important for pediatric sedation, paradoxically reactions. During paradoxically reactions associated with midazolam, the patient actually becomes excited rather than calm and relaxed; notable clinical changes include agitation, irritability, hyperactivity, rage, and hostility even toward the caregiver. The delayed paradoxical reaction typically becomes apparent within 30–40 min after its administration and lasts 1–3 h.
Midazolam is a good agent for short dental procedures such as a simple primary tooth extraction, but longer procedures may not be tolerated well by the child and disruptive behaviors may dominate. In a minority of cases, the disruptive behavior progresses to frank agitation and expressions of anger directed at anyone near the child including the parent as mentioned previously. This drug-related response has been called by various names, but may be characterized as the “angry child response” [5]. No definitive evidence is available for the number of children sedated with midazolam that exhibit this state, and likewise, little documentation for the duration of this response is readily available. It is the author’s opinion that this angry child response occurs in approximately 20 % and lasts for 1–2 h. There is some evidence that this response tends to be inversely related to the age of the child (e.g., more frequent in 2-year-olds) [6]. Also, some evidence exists to suggest that flumazenil will reverse the paradoxical reaction [7]. Another infrequent finding associated with midazolam when used alone is the presence of hiccups. The hiccups are not clinically significant and tend to disappear along with other effects as the drug is metabolized.
Diazepam and triazolam are also two other benzodiazepines used with children. Little evidence exists to suggest significant differences in the occurrence of sedated behaviors as a function of these agents. However, subtle timing differences suggest that the onset and length of working time may be slightly enhanced for diazepam and triazolam compared to midazolam. Hence, for longer dental procedures in older preschoolers and school-aged children, it is possible that diazepam and triazolam may be preferred over midazolam. Bouts of agitation can also occur with these two agents similar to that of midazolam. Some benzodiazepines come in a single-dose oral formulation (Fig. 4.4).


Fig. 4.4
Example of single dose delivery package of diazepam
Flumazenil is a benzodiazepine receptor antagonist. Flumazenil which is approved for intravenous administration only can reverse therapeutic doses of benzodiazepine effects including sedation within a minute or so. If flumazenil is administered by another parenteral route (e.g., submucosal), the duration required for reversal effect onset will be longer and may require intermediate, adjunctive interventions in an adverse situation (e.g., bag valve mask for respiratory depression or apnea). Furthermore, flumazenil apparently may not last as long as the benzodiazepine is being reversed; hence re-sedation effects can occur. Because of the possibility of re-sedation following reversal in hospital settings, administration of reversal agents generally requires a longer postoperative care monitoring period before discharge occurs. Flumazenil can also reverse some paradoxical reactions to benzodiazepines [8].
Meperidine
Meperidine is a synthetic opioid agonist with analgesic and atropine-like properties that has been popular among pediatric dentists for years. The gold standard for opioid agonists is morphine. Apparently there is no difference in behaviors in children sedated for dental procedures comparing morphine to meperidine [9]. Meperidine is approximately 1/10th the potency of morphine; however, it is equivalent to morphine in terms of the degree of sedation and respiratory depression. The duration of action of meperidine is less than that of morphine, and its oral effectiveness is significantly reduced compared to parenteral doses due to its first pass through the liver.
Meperidine administered in high doses can cause CNS tremors and convulsions. This effect is due to normeperidine, produced by demethylation of meperidine in the liver. In fact there is some evidence that high doses of meperidine interacting with local anesthetic doses that are high or exceed therapeutic values can induce seizures followed by respiratory depression and coma [10]. Meperidine is metabolized in the liver and excreted through the kidneys. As an opioid agonist, meperidine is thought to function by binding to opioid receptors in the CNS, primarily mu (μ) and k (κ) to inhibit ascending pain messages and modify affect related to pain.
Clinical
Meperidine when used to sedate children in the dental setting is usually administered by the oral route. Onset of effects can be appreciated in 20–30 min, and working time may extend upwards to 45 min to an hour assuming good behavior guidance is also applied.
Another route that is less popular is that of the submucosal injection similar to the infiltration of local anesthesia in the maxillary buccal vestibule adjacent to the first or second primary molar. The onset via this route is faster (i.e., 10–15 min), and the working time is essential the same as when given orally. Care must be exercised when administering submucosally, as sudden hypotension and respiratory depression can occur rapidly if inadvertently injected into the pterygoid plexus behind the maxillary tuberosity in children.
Meperidine can cause localized release of histamine when injected submucosally causing redness and itching over the malar area. It is thought that meperidine when administered orally may cause itching in and around the facial area; thus occasional rubbing of that area by the dentist in a restrained patient is recommended. Opioids, including meperidine, directly stimulate the chemoreceptor trigger zone in the medulla causing nausea and emesis which are not beneficial as a single agent in sedating children. Because of this side effect, hydroxyzine which has antiemetic effects is often administered with meperidine. Precaution is advised though as this combination of agents, like most, may increase the depth of sedation.
Clinically, the advantages of meperidine for sedating children during dental procedures are best appreciated as mediating mood changes and providing mild analgesia. The mood changes typically are euphoric-like in the majority of patients; however, a small proportion of children may have the opposite effect of dysphoric mood changes that compromise the outcome of sedation procedures. One must always be vigilant of the amount of local anesthetic used during sedations especially when multiple sedative agents are used. Since meperidine can add to the analgesic effect of local anesthesia, it is possible to use little or no anesthetic for doing simple and shallow class I preparations on primary molars.
Combining meperidine with midazolam can be beneficial in that the primary effects of each may produce a better clinical outcome. Anxiolysis, relaxation, a mellow mood, and increased analgesic effects may be possible when the doses of each agent are appropriate. Increased effectiveness has been suggested in one study when this combination is compared to midazolam alone [11]. It is important to note that higher doses of this combination can cause somnolence or deep sedation potentially compromising functional airway competency and endangering patient safety. Meperidine and midazolam can both be reversed with naloxone and flumazenil, respectively, with intramuscular administrations in the patient’s thigh. Advanced management of a compromised airway though may be necessary under these circumstances because of the longer onset of action associated with the intramuscular versus intravenous administration of the reversal agents.
Meperidine can also be administered with other sedatives to complement effects. For instance, a very effective combination of agents for older preschoolers (i.e., 3–5 years) is a low dose of chloral hydrate (10–20 mg/kg), meperidine (1–2 mg/kg), and hydroxyzine (1 mg/kg). This “triple” combination produces moderate sedation in most children of this age wherein ptosis or closure of the eyelids occurs, but the child remains responsive to verbal commands or very light physical stimulation. Some children may enter into deep sedation with this combination, and thus appropriate monitoring and management via training is required. Higher doses of chloral hydrate in this combination can rapidly lead to deep levels of sedation, and caution in its use is highly advised as the only reversible agent is meperidine.
Chloral Hydrate
Chloral hydrate is one of the oldest sedatives used for dental sedation. It has been very popular in pediatric dentistry since the mid-1950s. Chloral hydrate is classified as a sedative-hypnotic and is known to induce sleep in children. Chloral hydrate is rapidly absorbed following oral administration and is converted through its first pass in the liver to trichloroethanol, its active form. Trichloroethanol is conjugated in the liver and excreted in the urine. Like other agents that are metabolized in the liver, chloral hydrate may interact with other drugs, herbs, or foods resulting in clinically significant alterations of the agents (e.g., warfarin).
Trichloroethanol is a CNS depressant. Its mechanism of action is not fully understood; however, studies have shown that chloral hydrate, α-chlorose, and trichloroethanol interact with the GABA receptor complex, specifically the GABAA subunit which is also activated by benzodiazepines [12]. Hyperpolarization occurs as a result of increased Cl− conductance; hence inhibitory action is noted. Again, like the benzodiazepines, it is possible that chloral hydrate affects other neurotransmitter activating complexes, and the range of clinical effects witnessed may be expressed through the configurations of active receptor complexes throughout the CNS.
Chloral hydrate is a mucosal and gastric irritant. Its used is contraindicated in patients with esophagitis, gastritis, and duodenal inflammation. Choral hydrate has also caused laryngospasms and cardiorespiratory arrest after its aspiration [13]. Care must be taken that choral hydrate is not splashed into the eyes of the child or the individual administering chloral hydrate. In higher doses, chloral hydrate has been known to cause cardiac arrhythmias requiring immediate medical intervention. Chloral hydrate when administered with other sedative agents produces deeper levels of sedation than when either is used alone.
Clinical
Clinically in therapeutic doses (20–50 mg/kg), one typically sees a slight or even prominent hyperexcitability stage within 20–30 min after the administration of chloral hydrate. The hyperexcitability in children is manifested as hyperactivity, talkativeness, and frank change from shyness to friendliness. This stage usually lasts 20–30 min, before sleepiness begins to dominate, especially when higher doses of chloral hydrate are used (i.e., 40–50 mg/kg). Sometimes the hyperexcitability stage does not wane and makes the performance of dentistry challenging if not impossible.
Chloral hydrate is rarely administered alone anymore to children who undergo dental procedures. It is usually mixed with hydroxyzine which aids in minimizing vomiting and to deepen the level of sedation. Another popular “cocktail” is chloral hydrate with meperidine and an antiemetic such as hydroxyzine. This “triple” cocktail can readily produce deep levels of sedation and caution is advised. A “light” triple cocktail involves very low doses of chloral hydrate (10–20 mg/kg). This lighter version significantly lessens the depth of sedation, but still can cause unconsciousness in some children. In fact, whenever any sedative agent is combined with another, it is advisable to decrease the therapeutic dose of each agent.
The production of the oral solution of chloral hydrate was discontinued in 2012, limiting its availability. Compound pharmacists can still make a compounded solution of chloral hydrate but not on a volume basis (i.e., has to be prescribed and compounded, individually, for each patient).
It is thought that chloral hydrate does not have a reversal agent. Nonetheless, one clinical report did indicate that flumazenil reversed respiratory depression and hypotension in a chloral hydrate overdose situation involving a young man [14]. Regardless, one should never rely on this possibility in preplanning sedations with chloral hydrate.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


