and Armin Segarra1
(1)
University of the Philippines Manila College of Dentistry, Paranaque, Philippines
2.1 Introduction
The resin cements are the newest type of cements for indirect restorations, and they have the ability to bond to the tooth structure and the internal surface of the restoration. Resin cements are composed of the same basic component as the composite restorative material but with lower concentration of filler particles (Simon and Darnell 2012). These cements have higher compressive, flexural, and tensile strength than the conventional cements and can be used for almost any type of restoration and restoration material. These cements however are more complex than the conventional cements and are highly technique sensitive.
To maximize the properties of resin cements, a clear understanding of the factors that affect its clinical performance is of paramount importance. These factors are interrelated. The most important factor affecting the success of resin cements is the bond strength of the resin cement. Bond strength in turn is affected by pretreatment procedures, the depth of cure and degree of polymerization of the resin cement, and incompatibilities between the adhesive resin and the resin cement. Factors that may affect polymerization include cement film thickness, opacity, and translucency of both the cement and restoration and shade of the restoration. A properly cured resin cement will exhibit high compressive and flexural strengths, properties that enhance bond strength. Properly cured resin cements are also virtually insoluble to oral fluids. The mode of delivery and method of mixing the resin cement are also factors that may affect the overall clinical performance of the resin cement.
Understanding how all these factors are interrelated will minimize errors and enhance the longevity of bonded indirect restorations. This is intelligent cementation.
2.2 Pretreatments Prior to the Cementation Procedure
The resin cement bonds the underlying tooth structure to the internal surface of the restoration. Regardless of the type of resin cement, a bond should exist between the dentin and the cement (tooth-cement interface) and between the cement and the internal surface of the restoration (cement-restoration interface) (Fig. 2.1). For these bonds to form, the tooth and the internal surface of the restoration should be pretreated.
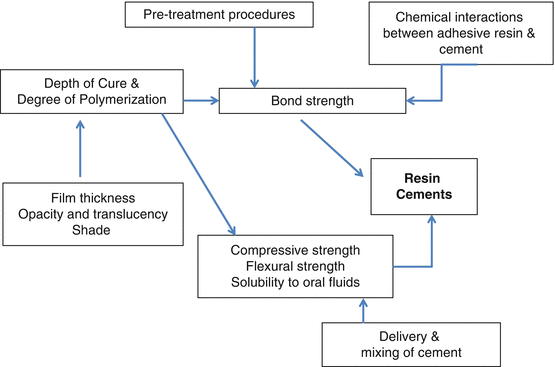

Fig. 2.1
Factors affecting the clinical performance of resin cements
2.2.1 Pretreatment of Tooth Structure
Resin cements mainly adhere to the tooth structure through micromechanical retention. To achieve this micromechanical retention, the usual adhesive steps of etching, priming, and bonding should be performed on the enamel and dentin to form a stable hybrid layer. Most resin cement systems come with their proprietary adhesives to avoid incompatibilities between adhesives and cements. Some cements use etch-and-rinse adhesive systems (etch-and-rinse or total etch resin cements), while other cements use adhesives containing self-etch primers (self-etch resin cements). Newer resin cements, the so-called self-adhesive resin cements, have their monomers and adhesives incorporated in the cement itself eliminating the need for pretreatment procedures. As cements adhere to tooth structure through resin bonding, care should be taken that the bonding substrates are clean and free from fluid contamination.
2.2.2 Pretreatment of the Internal Surface of the Restoration
The cement serves as a bridge between the tooth and the restoration. While tooth bonding procedures ensure that the cement adheres well to the tooth, pretreatment of the internal surface of the restoration ensures that the cement will adhere to the restoration as well. A good adhesion to the internal surface of the restoration requires (1) roughening of the internal surface of the restoration to increase the surface area for bonding and (2) increasing the wettability of the cement to the restoration and forming chemical bonds between the ceramic, the fillers, and the cement. Depending on the restoration material, the first procedure is done through air abrasion, sandblasting, or etching with a hydrofluoric acid (for ceramic and composite restorations) or application of an alloy primer (for restorations with a metal subsurface).
The second procedure is achieved by applying a silanating agent on the etched porcelain or composite. The silane makes the ceramic chemically adhere to the resin cement through covalent and hydrogen bonds (Horn 1983). Silanating the internal surface of indirect composite restorations ensures that the fillers of the composite react and adhere with the resin cement (Calamia and Simonsen 1985).
As restoration pretreatments differ from material to material, knowledge of the different types of tooth-colored materials (composites and ceramics) used in dentistry can simplify pretreatment procedures for tooth-colored indirect restorations.
2.3 Classification of Dental Ceramics
There are different ways of classifying ceramics or different terms for different types of ceramics. To simplify it, dental ceramics can be classified into two broad groups based on their composition: the silica-based ceramics and the non-silica-based ceramics (Blatz and Kern 2003). Since the physical and mechanical properties of ceramics depend mainly on their composition, silica-based ceramics are also referred to as low-to-moderate-strength ceramics, and non-silica-based ceramics are the high-strength ceramics. Based on their structural component and phases, silica-based ceramics are also called glass-ceramic systems, and non-silica-based ceramics are called polycrystalline ceramics. The silica-based ceramics are further classified into feldspathic porcelains, leucite-reinforced ceramics, and lithium disilicate ceramics (Table 2.1).
Table 2.1
Classification of dental ceramics
|
Classification
|
Subtypes
|
Represenatative brands
|
Flexural strength
|
Main feature
|
Indications
|
|---|---|---|---|---|---|
|
Silica-based ceramics (aka: glass-ceramic systems; low-moderate strength ceramics; 1st generation ceramics ceramics
|
Feldspathic porcelain (aka veneering porcelain)
|
CEREC Blocs, Eris, Kiss, Classic, LavaCeram, Creation
|
65–120 MPa
|
High translucency, very esthetic
|
Veneers
|
|
As a veneering layer for high strength core ceramics
|
|||||
|
Should not be used when there is discoloration or masking is an issue
|
|||||
|
Leucite-reinforced ceramic
|
IPS Empress
|
120–140 MPa
|
Highly esthetic
|
Anterior crowns
|
|
|
Inlays and onlays
|
|||||
|
Leucite crystals act as crack deflectors to increase resistance to crack propagation
|
As a layering porcelain on high strength ceramic cores
|
||||
|
Lithium disilicate
|
E-max
|
300–400 MPa
|
High strength with good esthetic
|
Vaneers
|
|
|
Inlays and onlays
|
|||||
|
Posterior crowns
|
|||||
|
3-unit bridges (anterior and premolar region)
|
|||||
|
Non-silica based ceramics (high-strength polycrystalline ceramics)
|
Alumina
|
Porcera
|
650 MPa
|
High strength
|
Inlays and onlays
|
|
Posterior crowns
|
|||||
|
3-unit bridges
|
|||||
|
Zirconia
|
Lava
|
800–1,500 MPa
|
Superior strength
|
Anterior and posterior crowns
|
|
|
Cercon
|
Anterior and posterior bridges
|
||||
|
CERE in Lab
|
Inherent opacity
|
Endodontically treated teeth
|
|||
|
InCeram Zirconia
|
Randomized clinical trials and clinical experience have been controversial regarding long-term survival a
|
Maryland bridges (bonding might be a problem)
|
|||
|
IPS emaxZirCAD
|
Implant abutments
|
||||
|
Katana
|
Inlay bridges
|
||||
|
Procera AllZirkon
|
Block-out of darkened tooth structure or cores
|
2.3.1 Pretreatment for Ceramics Based on Their Classification
Dental ceramics, because of their differences in composition and phases, therefore require different pretreatment procedures. Silica-based ceramics will require either etching with hydrofluoric acid or sandblasting and subsequent silanization to improve adhesion to the resin cement.
Hydrofluoric acids (HF) roughen the internal surface of the restoration. They are available in varying concentrations from 2.5 to 10 %, and etching time is usually 2–3 min (Chen et al. 1998). Etching ceramic with hydrofluoric acid renders the surface microscopically porous, increases the surface energy resulting in a microretentive surface (Hussain et al. 1979; Suliman et al. 1993). Care should be taken not to over-etch the porcelain with hydrofluoric acid as it can weaken the bond between the ceramic and resin cement. After HF etching, a white residue sometimes forms on the surface of the porcelain. This white residue is a potential contaminant and should be removed prior to silane application. Recommended methods of removing this residue include immersing in an ultrasonic cleaner for 5 min, steam cleaning, or using an alcohol solution (Alex 2008).
Silane-coupling agents, or simply silane, ensure a good bond between the hydroxyl groups of the ceramic and the organic portion of the resin cement. They are available in two forms (Manso et al. 2011): (1) pre-hydrolyzed single-bottle solutions or (2) two-bottle solutions. Silanes have a rather short shelf life and, once exceeded, are virtually ineffective and unusable. Clinically, a milky-colored solution indicates that the silane is well past its expiration date and should be discarded (Blatz and Kern 2003). This is especially true for the two-bottle systems. Unfortunately, since one-bottle silanes are alcoholic, they stay transparent which makes it difficult to gauge whether they are still usable. Clinicians should strictly respect expiration date and follow manufacturer’s instructions when using silanes.
The silane is applied on the internal ceramic surface and then air-dried. There is no consensus on the duration of silane application as it may range from 5 min to 2 h. The usual application time is between 60 and 90 s (Anagnostopoulos et al. 1993; Martinlinna et al. 2004; Alex 2008). This application forms the so-called interphase layer, which is actually three layers. The outermost layer and the middle layer are hydrolyzable and can adversely affect adhesion of the ceramic to the resin cement. These two layers should be removed. The innermost layer, closest to the internal surface of the restoration, is a monolayer, which is chemically bonded to the silica phase of the ceramic and is actually responsible for adhesion to the resin cement.
The silanated ceramic should appear dull and not shiny. A shiny surface is indicative of excessive silane and can affect the bond of the ceramic to the resin cement. The silanated surface is then air-dried preferably with warm air. This method of drying, together with the contaminants during the try-in procedure, usually removes the hydrolyzable outermost and middle layers (Ishida 1985).
One important thing to remember is that a hydrofluoric acid-etched ceramic is very prone to contamination with oral fluids. The laboratory usually does the hydrofluoric acid etching. During try-in, the hydrofluoric acid-etched ceramic restoration can be contaminated with saliva. One suggestion to prevent contamination is to apply the silanating agent immediately after hydrofluoric acid etching and prior to try-in as the silane renders the etched ceramic hydrophobic and thus more resistant to fluid contamination. Another advantage of silanating prior to try-in is that the try-in procedure removes the hydrolyzable outermost and middle layers of the silane, rendering the internal surface more conducive to bonding with the resin cement (Manso et al. 2011).
Non-silica-based ceramics such as alumina and zirconia have polycrystalline phase and should not be etched as they are highly resistant to chemical attack from HF (Sorensen et al. 1991; Valandro et al. 2005; Ozcan and Vallitu 2003) or silanated as it might destroy the crystalline structure and weaken the material. Other studies find no improvement in adhesion when alumina and zirconia are etched and silanated prior to cementation. This explains why achieving high and durable bond strengths to alumina and zirconia ceramics is difficult.
The preferred pretreatments for alumina or aluminum oxide ceramics include (1) airborne abrasion with 50–110 μm aluminum oxide particles at 2.5 bars, (2) use of an MDP-containing resin cement (Panavia 21, Kuraray, Japan; Single Bond Universal (3 M Espe, Germany)), or (3) silicoating through tribochemical surface treatment (Rocatec, 3 M Espe, Germany) followed by application of a conventional bis-GMA resin cement (Blatz and Kern 2003; Kern et al. 2009; Kitayama et al. 2010; Yun et al. 2010).
Several surface treatments have been studied to improve bonding with zirconia ceramics. These include APA (airborne particle abrasion) or wet hand grinding and tribochemical silicoating. APA or wet grinding roughens the surface of the zirconia which was thought to improve bonding. However, some studies have shown that grinding or sandblasting may create surface defects and sharp cracks that render the zirconia prone to cracking or fracture during function (Zhang et al. 2004). Tribochemical silicoating was introduced in an attempt to improve bond without compromising the physical and mechanical properties of zirconia (Kern and Thompson 1994; 1995). In tribochemical silicoating, the internal surface of the zirconia is air abraded with aluminum trioxide particles with silica to coat the zirconia with silica aluminum. This renders the internal surface of the restoration chemically adhere to the resin cement. Studies done on tribochemical silicoating however showed decreased bond strengths with resin cements during aging and thermocycling (Kern and Wegner 1998; Wegner and Kern 2000; Ozcan and Vallitu 2003).
Resin cements and primers containing the acidic monomer 10-MDP are the recommended cements for zirconia ceramics as MDP can chemically bond with zirconia (Tanaka et al. 2008; Oyague et al. 2009). Examples of such cements and primers are Panavia F 2.0, SE Bond, SA Luting Cement (Kuraray, Osaka, Japan) and the newer Scotchbond Universal adhesive (3 M Espe, Germany). Aside from these 10 MDP-containing primers, primers such as Metal/Zirconia Primer (Ivoclar), Z-Primer (Bisco), and AZ Primer (Shofu) which contain phosphoric acid monomers can also be used to promote the adhesion of alumina and zirconia due to chemical bond formation (Kern et al. 2009; Kitayama et al. 2010).
Indirect composite or laboratory composites were developed in an attempt to improve on the physical and mechanical properties of direct composites as well as facilitate carving of adequate proximal contours and contacts and occlusal anatomy. Indirect composites have microhybrid fillers and are highly filled with less of the organic matrix to minimize polymerization shrinkage (Nandini 2010). This class of composites undergoes secondary curing either by heat polymerization or high-intensity light polymerization. Secondary curing has been found to decrease bonding of the restoration to the resin cement as secondary curing leaves no available monomer for subsequent bonding to resin cements (Kildal and Ruyter 1994). Suggested pretreatments for indirect composites include sandblasting followed by phosphoric acid etching the internal surface of the restoration. The sandblasting roughens the internal surface, while phosphoric acid etching cleans the sandblasted surface of debris (Jivraj et al. 2006
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


