Fig. 4.1
Root canal anatomy of maxillary first molar and the effects of instrumentation as revealed by micro-computed tomography. The preoperative canal system is shown in red; the post-instrumentation shape of the canal is indicated by green (Courtesy “Visual Endodontics/Artendo Enterprises Inc.”)
Models to Study Cleaning of Isthmus Areas
Modern instruments and instrumentation techniques are able of reaching all irregularities of the root canal system. Therefore, dentists must irrigate the uninstrumented areas to remove debris and biofilms. Isthmus areas (connections between two root canals in the same root) in posterior teeth are challenging to irrigate, which may result in survival of microorganisms and only partial removal of dentin debris and tissue remnants. The incidence of canal isthmi varies depending on the type of tooth [76], root level [6], and age [77]. In one study the prevalence of isthmi ranged from 17 to 50 % in the apical 5 mm of the mesial root of mandibular first molars, with the highest prevalence at the 3-mm level [78]. Another study [77] showed that the highest prevalence of isthmus in mandibular first molars is 4–6 mm from the apex.
Use of micro-CT in endodontic research has made it easier to study the effects of instrumentation and irrigation in the root canal system. Recently, a method was presented to quantitatively assess accumulation and removal of inorganic debris in molar teeth instrumentation and irrigation [79, 80]. However, limitations of the micro-CT include that it only can be used on extracted teeth and that it can detect inorganic but not organic matter. Consequently, the chemical effects on soft tissues by NaOCl cannot be measured. A study evaluated the packing of hard tissue debris into isthmus areas of mesial roots of mandibular molars using rotary ProTaper instruments without any irrigation [79]. It showed that ca. 30 % of the original canal system was filled with hard tissue debris after preparation. The study emphasized that debris accumulation can be an undesired consequence of instrumentation. Such packed debris may have a negative impact on the sealability of root canals and reduce the effectiveness of disinfection. Even copious irrigation during and after instrumentation was not able to prevent or remove the debris packed into the isthmus area between the main root canals [80]. Thus, despite rigorous irrigation, the accumulation of dentin debris seems to occur and restrict cleaning and disinfecting the areas blocked by the debris (Fig. 4.2).
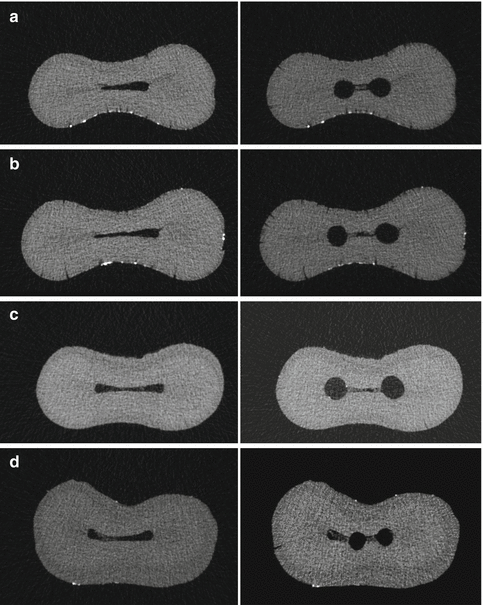

Fig. 4.2
Micro-computed tomographic cross sections of mesial root canals of four mandibular molars treated with rotary NiTi instruments (a–d). The cross sections are shown before instrumentation (left) and after instrumentation (right). Note the presence of accumulated hard tissue debris in the ribbon-shaped isthmus area after instrumentation (the four cross sections on the right)
In an in vivo situation, the canal is like a closed-end channel, which often results in gas entrapment and a vapor lock effect at its apical end [84–86] during irrigation [12, 81–89]. Studies designed to simulate a closed root canal system have demonstrated incomplete debridement from the apical part of the canal walls with the use of a syringe delivery technique [90–92]. Johnson et al. [93] compared debridement efficacies of a sonic irrigation technique (Vibringe; Cavex Holland BV, Haarlem, the Netherlands) with side-vented needle irrigation (SNI) in the mesiobuccal root of maxillary first molars using a closed canal model. The tooth selection in this study was that the mesiodistal isthmus width of completely patent isthmi or partially obliterated isthmi had to be less than one-quarter of the diameter of the unshaped canals along the canal levels (i.e., 1–2.8 mm from the anatomical apex) from which histological sections would eventually be prepared after completed chemomechanical preparation. Histological sections showed that neither technique could completely remove the debris from the canal or isthmi. A significant difference between the two methods was only identified between the canals and the isthmi. Both instrumented canal spaces and uninstrumented isthmus regions are cleared of soft tissue debris to the same extent using the sonic irrigation device or the conventional SNI technique.
The presence of a complex, variable, multi-species biofilm was recently demonstrated in the entire length of the isthmus of a tooth, which had initially been treated 10 years earlier and then re-treated 2 years later [94]. Gram-positive and Gram-negative organisms were both detected. In light of the well-documented challenges in obtaining the desired cleanliness, this area can have a negative impact on the long-term prognosis of non-surgical endodontic treatment.
Dentin Canals
The bulk of root dentin is traversed by the dentin canal (dentinal tubules). Bacteria have been shown to be present in dentinal tubules in most teeth with apical periodontitis [95–97]. Several different approaches have been used to study the effect of irrigation on microbes inside the dentin canals. Ørstavik and Haapasalo [98] investigated the effect of endodontic irrigants and locally used antibacterial agents in standardized bovine dentin blocks infected with test bacteria. The authors reported that bacteria colonized the main root canal lumen and dentin canals. E. faecalis infected the entire length of the tubules, whereas Escherichia coli penetrated approximately 600 μm. Some other studies have shown that bacteria can penetrate dentinal tubules to depths of 200 μm or more [99, 100] (Fig. 4.3). Mechanical cleaning/disinfection means the removal of some of the infected root canal wall dentin. However, complete uniform enlargement of a root canal by 200 μm is not achieved with any of the contemporary instruments [101, 102]. Berutti et al. [103], using bacterial culture from dentin samples, showed that irrigating the canal with sodium hypochlorite (after removing the smear layer) rendered the dentinal tubules bacteria-free only to a depth of 130 μm from the canal lumen.
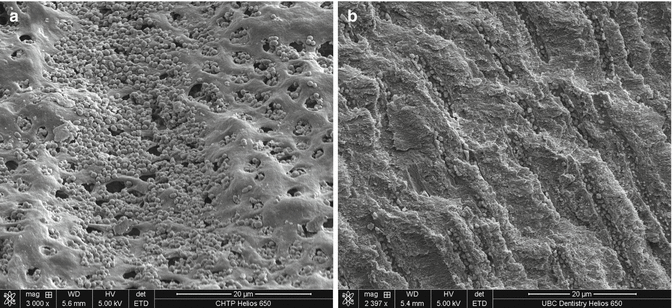

Fig. 4.3
A scanning electron microscope (SEM) image of Enterococcus faecalis in dentinal tubules in cross-sectional (a) and longitudinal (b) view (Courtesy “Visual Endodontics/Artendo Enterprises Inc.”)
Berber et al. [54] investigated the efficacy of 0.5, 2.5, and 5.25 % sodium hypochlorite as intracanal irrigants associated with hand and rotary instrumentation techniques against E. faecalis within root canals and dentinal tubules. The samples collected from the root canals with paper points were obtained just after biomechanical preparation in order to evaluate the chemicomechanical action immediately after the instrumentation. The dentin samples were obtained using burs of different diameters in order to evaluate the presence of bacterial cells inside the dentinal tubules following the biomechanical procedures. The samples obtained with each bur were placed into brain–heart infusion (BHI) broth, incubated at 37 °C, and plated onto BHI agar. The results indicated that instrumentation and irrigation with saline only removed more than 95 % of the bacterial cells from the root canal. At all depths of the root canals and for all techniques used, 5.25 % NaOCl was shown to be the most effective irrigant solution tested when dentinal tubules were analyzed, followed by 2.5 % NaOCl. No differences between the different hypochlorite concentrations in cleaning the main root canals were found. Although dentin in most teeth with apical periodontitis is infected by bacteria invading from the main root canal, histological sections stained with the Brown and Brenn method and SEM studies have both shown that bacteria are found only in a few dentinal tubules even after a prolonged period of incubation [98, 104]. Such a low level of dentin infection makes it difficult to reliably measure the effects of disinfecting agents by culture or by confocal laser scanning microscopy (CLSM). Therefore, a dentin model that allows predictable, dense, and deep penetration of bacteria would be most useful for the study of endodontic disinfection [100, 105]. Recently, a standardized three-dimensional in vitro model for quantitative assessment of bacterial viability in dentin by CLSM after infection and disinfection of the dentinal tubules was developed [64].
The effect of concentration, time of exposure, and temperature on the penetration of NaOCl into dentinal tubules was recently studied [106]. The depth of penetration of NaOCl was determined by the bleaching of the stain and measured by light microscopy. The results showed that the ability of sodium hypochlorite to penetrate dentinal tubules was dependent on time, concentration, and temperature, but the relative effect of the three factors was much smaller than expected. For instance, penetration after 20-min exposure was only twice (not ten times) as much as after 2-min exposure, and the differences between penetration by 1 and 6 % NaOCl were rather small (Fig. 4.4). Maximum penetration of 300 μm was seen when 6 % sodium hypochlorite was used for 20 min at 45 °C in coronal and mid-root dentin.
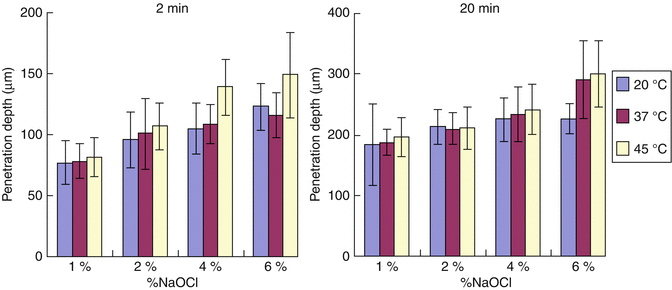

Fig. 4.4
Depth of penetration (in vitro) of sodium hypochlorite in various concentrations and at different temperatures into dentin canals in 2 min (left) and 20 min (right)
Several studies have reported that dentin weakens the antibacterial effectiveness of calcium hydroxide, iodine potassium iodide, and sodium hypochlorite [32, 33]. The survival of the bacteria could therefore also be attributed to their invasion into the dentinal tubules where they are better protected from endodontic medicaments than in the main canal. This may be caused by the difficulty of the solutions to penetrate into the tubules, inactivation of the medicaments by dentin, or the microbial biomass in the tubules [33]. During chemomechanical preparation of the root canal, use of chelating agents and acids results in selective removal of inorganic dentin components, exposing collagen fibers. Portenier et al. [34] studied the potential inhibitory effect of bovine dentin matrix (collagen), demineralized dentin powder (treated with EDTA or citric acid), and skin collagen on the antibacterial activity of 0.02 % CHX and 0.1/0.2 % iodine potassium iodide (IPI) solution. Dentin matrix (3 % w/v), which mostly consists of purified dentin collagen, was a potent inhibitor of both CHX and IPI, with most E. faecalis cells surviving after 24 h of incubation with the medicaments in the given concentrations. Dentin matrix was a slightly less effective inhibitor of IPI than dentin, but on CHX its effect was stronger than that of dentin. This is in accordance with earlier reports which have shown that IPI was more susceptible to dentin than to organic compounds, whereas the opposite was true for CHX [32, 33]. When EDTA or citric acid was first used to dissolve the apatite, dentin inhibited the activity of CHX more than untreated dentin powder but less than purified dentin matrix. No difference was detected between EDTA and citric acid treatment [34]. When IPI was tested, demineralized dentin (pretreated with EDTA or citric acid) showed no inhibitory activity. It can be speculated that rinsing with EDTA or citric acid before irrigation with disinfecting agents might weaken the effect of CHX but strengthen the effect of IPI. Comparative experiments have indicated that skin collagen is a weaker inhibitor of IPI and CHX than dentin matrix [34]. Together with the observation that dentin treated with EDTA or citric acid caused inhibition that was stronger than with skin collagen but weaker than with dentin matrix, this indicates that there are important differences between type I collagen products obtained from different sources and through different production and purification methods. In summary, dentin is a complex chemical and anatomical environment that needs to be carefully considered when designing studies looking at the effects of irrigation.
Lateral Canals
Accessory (lateral) canals branch from the main root canal, with diameters ranging from over 100 μm to a common minimum of 10 μm [107]. Such narrow orifices create a surface tension barrier that does not allow adequate mixing between the irrigant and the liquid within the canal. The narrowing of the root canal apically (toward the root) poses a similar barrier. Any fluid flowing down the accessory canals from the root canal will be laminar flow; turbulent flow will be not be achievable due to the very low Reynolds numbers inherent at such small “pipe” diameters, where edge effects and viscosity become the major factors affecting fluid dynamics [76, 108]. The lateral canals may contain bacteria/bacterial biofilm which may cause lateral, periradicular bone lesions. Histological sections of extracted teeth have indicated that the lateral canals are not completely cleaned and, after root filling, they often still contained vital or necrotic pulp tissue and bacteria [109]. As long as there is no method to completely and predictably clean and disinfect lateral canals, microbes in the lateral canals remain one possible reason for posttreatment endodontic disease.
The small number of studies on irrigant action in lateral or accessory canals is probably due to the difficulty of such studies, as the accessory canal position and status before treatment are difficult to determine. Consequently, there is a need for standardized models that simulate accessory canals. Models of artificially created lateral canals in cleared teeth or an epoxy resin have recently been developed to evaluate efficacy of irrigant penetration [88, 110].
Smear Layer
Use of any kind of metallic instrument in the root canal results in a smear layer wherever the instrument comes into contact with the root canal wall [111, 112] (Fig. 4.5). Smear layer is a 1–2-μm-thick, amorphous, irregular, and granular layer with a deeper part that can penetrate up to 40 μm into the dentinal tubules. The penetration is hypothesized to be the result of capillary action and adhesive forces between the dentinal tubules and the smear layer [113, 114]. Others have estimated the layer to be up to 5 μm thick with inorganic particles of 0.05–0.15 μm diameter [115–117]. Essentially, the smear layer is a complex mixture of inorganic and organic particles, proteins, pulp tissue, blood cells, and, in infected canals, bacteria and fungi [118, 119]. As the irrigation needle is likely to follow the path created by the endodontic instruments, delivery of irrigants to areas covered by the smear layer is usually unproblematic except perhaps in the most apical canal. Irrigation with the needle introduced only to the coronal or middle parts of the root canal (needle too big in size or apical canal not sufficiently enlarged) will result in incomplete removal of the smear layer in the apical root canal.
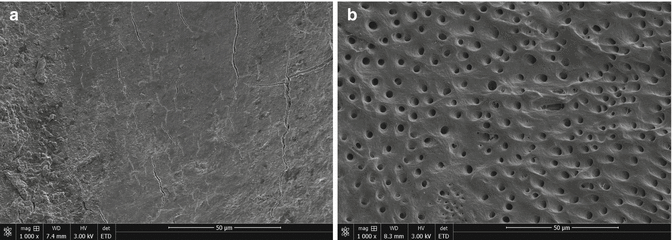

Fig. 4.5
Instrumented canal wall (a) with smear layer and (b) after removal of the smear layer by NaOCl and EDTA
Various methods have been used to evaluate the smear layer removal in vitro. These include score-based conventional SEM examination or optical microscopy techniques [120, 121]. However, the results obtained from score-based conventional SEM studies are not always reproducible. Therefore, further efforts must be directed to the development of, e.g., computational routines able to automatically extract quantitative data of dentin morphology, thus minimizing human bias. Calcium ions chelated from the root canal have been quantified by atomic absorption spectrophotometry [122, 123].
Therefore, the factors that remain a challenge in the irrigation and disinfection of the root canal include biofilm resistance [124, 125], irrigant penetration [39] and concentration [27], exposure time often very short [38, 39], small overall volume [126], and poor exchange of irrigants in the highly complex root canal system [107, 108]. Progress in the search for safe and more effective irrigant delivery and agitation systems for root canal irrigation is therefore necessary. Newer studies of irrigation have closely examined the same variables associated with irrigation efficiency, but unlike in the previous decades, these studies are increasingly utilizing novel experimental models. An improved understanding of the challenges by microbial biofilms by new research models and designs is likely to help us to better eliminate biofilm infections in the future.
New Models to Study Irrigation
Measuring Antibacterial Activity
Irrigation is complementary to instrumentation in facilitating the removal of pulp tissue and/or microorganisms. However, the available irrigants face great challenges in their effort to eliminate the biofilm from the root canal. Studying endodontic microorganisms adhered to surfaces for their response to antimicrobial agents, e.g., irrigating solutions, calls for relevant in vitro models. Therefore, many in vitro biofilm models have been developed for the testing of the antimicrobial effectiveness and strategies of irrigation. However, the testing of antimicrobial agents against bacteria in biofilms has not been standardized. Not surprisingly, activity of the same disinfectants shows considerable differences between studies and experiments, which may be attributed to the diversity of the microbial growth phase, biofilm models, and procedures utilized for the analysis. Therefore, a number of parameters need to be considered in the design of a representative biofilm model for application in irrigation studies.
Biofilm Substrate, the Surface to Attach to
The structure and susceptibility of biofilms to antimicrobials are affected by a number of factors such as the available nutrients and the substratum where the biofilm has attached to [41, 42]. The majority of endodontic studies on biofilm have been conducted by allowing cells to grow on membranes, glass, or plastic. This allows the film to be first grown on a substrate (e.g., membrane) and then removed and placed in a defined amount of the antimicrobial agent. It has been established that the development and structural organization of a biofilm are influenced by the chemical nature of the substrate [127]. Dentin is a composite material made up of an organic fraction (around 20 wt%), which is mainly collagen, and an interpenetrant inorganic fraction (around 70 wt%). The latter is composed primarily of hydroxyapatite (HA), which exists both within the collagen fibrils (intrafibrillarly mineralized) and between fibers (interfibrillarly mineralized) on a nanometric scale [128]. Type I collagen is the major organic component (90 %) of dentin, although small amounts of several non-collagenous proteins are also present in dentin. Certain bacteria can attach to type I collagen in dentin [97] through the expression of surface adhesins and form biofilms [129, 130]. Biofilm experiments on polycarbonate or glass, due to the different chemistry of the substrate, may not represent a true indication of the bacteria–substrate interaction. It has been reported that HA coated with type I collagen provided an excellent substrate for multi-species biofilm growth (Fig. 4.6) [39]. Chemical similarity with the teeth/dentin and the excellent growth of the multi-species biofilm indicate that this model has the potential to serve as a standard biofilm model for studies of in vitro endodontic biofilms. The abundant growth of oral spiral forms (Fig. 4.6) in this multi-species in vitro biofilm has not been described previously. More bacteria survived in the collagen-treated HA biofilm than in the HA model in the medicament groups and a thicker biofilm was observed (Fig. 4.7) [39, 42]. However, this or any other model does not simulate dentin microanatomy. On the other hand, the standard shape of the discs makes it possible to grow biofilms with consistent characteristics, which has proven difficult when using dentin as the biofilm substrate. However, it is important to keep in mind that several additional local factors in the root canal environment may affect the function of the various irrigating solutions. Therefore, conclusions from in vitro biofilm models must be drawn with caution.
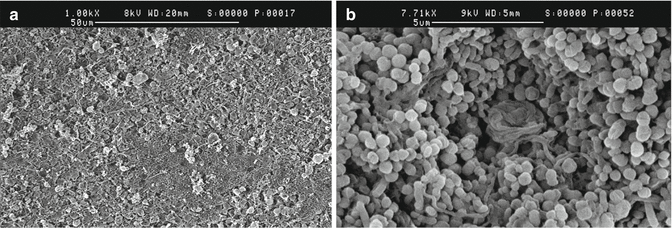

Fig. 4.6
(a) Scanning electron micrograph of a 3-week-old biofilm with mixed bacterial flora. (b) Several tightly coiled spiral forms which probably represent Treponema ssp. can be seen in the biofilm (Courtesy “Visual Endodontics/Artendo Enterprises Inc.”)

Fig. 4.7
Scanning electron micrograph of a cross section of 3-week-old biofilms. (a) Biofilm grown on the hydroxyapatite disc without collagen coating. (b) Biofilm grown on a hydroxyapatite disc coated with collagen
The biofilm substratum (surface where it is attached to) influences both the initial adhesion of the colonizing cells and the production of signaling molecules that control cell physiology and virulence. Chávez de Paz et al. [42] reported that biofilms formed on surfaces preconditioned with collagen showed a more patchy structure than those formed on clean polystyrene surfaces. These differences can be explained by a selection of cells that adhere exclusively to the weakly hydrophobic tracks created by surface oxidation on the collagen–substratum interface [131]. It is possible that such phenomena occurring at the collagen–substratum interface level may influence the stress response in biofilm bacteria when exposed to antimicrobials. In this study, Streptococcus gordonii, E. faecalis, and Lactobacillus paracasei showed a much higher number of viable cells after exposure to 1 % NaOCl on a collagen-coated surface than on an uncoated surface, although the proportion of removed cells was still high. The mechanisms behind these changes are not fully understood. The levels of dehydrogenase and esterase enzyme activities of biofilm cells on collagen-coated surfaces were much lower than on uncoated surfaces [42]. Such documented metabolic downregulation represents one possibility how the substrate surface condition may influence bacterial physiology.
Various hard tissues such as bovine teeth have been used in an attempt to find a replacement for human teeth in scientific research [132]. Lundström et al. [133] developed a “bovine tooth biofilm” model system and used this model to compare the bactericidal activity of concentrated stabilized chlorine dioxide with various concentrations of irrigants commonly used in endodontic treatment protocols. The teeth were coated with mucin; inoculated with standardized suspensions of Streptococcus sanguinis, Actinomyces viscosus, Fusobacterium nucleatum, Peptostreptococcus micros, and Prevotella nigrescens; and incubated anaerobically. Bovine dentin has a higher mean value of tubules per millimeter but the difference in the diameter of individual tubules is not significant [134]. Several studies have focused on dentin permeability [135–137] and effects of the therapeutic agents applied directly on the exposed dentin which may be dependent on the number and diameter of the dentin tubules [138, 139].
The “infected extracted tooth biofilm” model often uses a single-species biofilm on the root canal walls of extracted single-rooted teeth [45]. Bhuva et al. [46] grew E. faecalis biofilms on prepared root canal walls (for 72 h) of longitudinally sectioned, standardized root halves. Scanning electron microscopy was used to measure the effects of different irrigation protocols on the E. faecalis biofilms. However, as the length of incubation was only 2 days, the biofilms grown in this study are not as resistant as the true in vivo polymicrobial biofilms. Biofilms found in teeth with apical periodontitis are typically much older, with greater substrate adhesion and dentinal tubule penetration, and therefore much more resistant to the effects of chemomechanical treatment.
Surface modifications are known to prevent or reduce bacterial adhesion and biofilm formation by the incorporation of antimicrobial products into surface materials and by modifying the physicochemical properties of the surface [140–142]. Biofilm formation by oral bacteria after breakdown of temporary or permanent restorations is an unfortunately common challenge to the outcome of root canal treatment. Antibiofilm coatings can alter root canal surface properties and thus interfere with bacterial adhesion. Benzalkonium chloride (BAK) is a cationic detergent expressing a high affinity to membrane proteins. Its antibacterial potential relies on the changes provoked on the ionic resistance of the cell membranes [143]. It was recently reported [144] that a surface coating with a solution of BAK greatly reduced biofilm formation by oral bacteria in a dentin disc model and in an in vitro biofilm model.
Mono- and Multi-species Biofilms
Single-species biofilm models have been the most prevalent in endodontic and microbiologic research [145]. Spratt et al. [146] tested a variety of irrigants against five different facultative and obligate anaerobic single-species biofilms grown on membrane filter discs. Single-species biofilms of Prevotella intermedia, Peptostreptococcus micros, Streptococcus intermedius, Fusobacterium nucleatum, and E. faecalis were generated on membrane filter discs (incubated for 48 h in an anaerobic cabinet) and subjected to 15-min or 1-h incubation with colloidal silver, 2.25 % sodium hypochlorite, 0.2 % chlorhexidine, or 10 % iodine [146]. The results showed that the effectiveness of a particular agent was dependent on the type of organism and on the contact time. This model has the advantage of at least some level of standardization; it is easily reproducible and allows large quantities of test assays to be performed at one time. The limitations include lack of substrate similar to dentin and the limited number of different bacterial species. Short-term incubation for only 2 days is also a weakness of this model. In a similar study the effect of NaOCl and chlorhexidine on single-species biofilms grown for 10 days on nitrocellulose membranes was examined [147]. The organisms tested were facultative and anaerobic bacteria. The effect of mechanical agitation was also tested. The results indicated that both CHX and NaOCl were effective at killing all of the organisms tested, although the results varied with regard to time, vehicle, concentration, and mechanical agitation of the irrigant. Mechanical agitation improved the antimicrobial properties of the chemical substances tested using a biofilm model. However, compared to Spratt et al. [146], in this study the biofilm has been grown for ten instead of 2 days, which may explain the greater biofilm resistance.
Bryce et al. [148] investigated the relative disruption and bactericidal effects of root canal irrigants on single- and dual-species biofilms of root canal isolates. Biofilms of S. sanguinis, E. faecalis, F. nucleatum, and Porphyromonas gingivalis were grown on nitrocellulose membranes for 72 h and exposed to NaOCl, EDTA, chlorhexidine, or iodine for 1, 5, or 10 min. The organisms in the dual-species biofilms included S. sanguinis and F. nucleatum. The ratio of each organism was 1:2 (absorbance of 0.2 and 0.4 at 540 nm) for the S. sanguinis and F. nucleatum, respectively, and these were incubated anaerobically. The Gram-negative obligate anaerobe species were more susceptible to cell removal than Gram-positive facultative anaerobes. The majority of the cells were killed after the first minute of exposure; however, the extent varied according to the agent and species. Biofilm disruption and cell viability were influenced by the species, their co-association in dual-species biofilms, the test agent, and the duration of exposure. Jiang et al. [149] also investigated a root canal disinfectant on dual-species biofilms. E. faecalis with or without Streptococcus mutans in biofilms were formed in an active attachment biofilm model for 24 h. This model consisted of a standard 96-well microtiter plate and a lid with an identical number of polystyrene pegs that fit into the wells [150, 151]. The biofilms were then treated with various concentrations of NaOCl for 1 min. The resistance of dual-species biofilms to NaOCl was 30-fold higher than in single-species E. faecalis biofilms. The resistance to NaOCl of single-species S. mutans biofilms was comparable to that of the dual-species biofilms. The maturation status of the cells in biofilms is a possible reason for their higher resistance [152]. It is also possible that the antimicrobial resistance is related to the amount of biofilm biomass rather than the bacterial interactions in the biofilms. Single-species E. faecalis biofilms contain less biomass than the single-species S. mutans biofilms and the dual-species biofilms, which may explain the highest sensitivity [153]. Recently, Du et al. [154] evaluated the in vitro killing activity of modified nonequilibrium plasma with CHX against E. faecalis and multi-species biofilms on bovine dentin discs. Sterile bovine dentin discs were incubated with E. faecalis or a mixture of bacteria from human dental root canal infections to form 1- and 3-week-old biofilms. The results showed that there were only small differences in the susceptibility between the single-species E. faecalis biofilm and the multi-species biofilm. This may also be regarded as an indication that biofilm features such as maturation and extracellular polymeric substance are more important in determining the biofilm resistance than its detailed composition.
The development of in vitro multi-species biofilm models is challenging. However, they are necessary to better simulate interactions that take place, e.g., in root canal biofilms. Over the past years, biofilm research in endodontics has used both single-species [155, 156] and multi-species models [39, 157]. Chávez de Paz [158] investigated the ability of four root canal bacteria to establish a multi-species biofilm community and to characterize the main structural, compositional, and physiological features of their communities. The clinical isolates from infected root canals included Actinomyces naeslundii, Lactobacillus salivarius, Streptococcus gordonii, and E. faecalis which were grown together in a miniflow cell system. Suspensions of the four microorganisms were mixed in equal proportions to create the mixed-species biofilm inoculums. The species tested were able to form stable biofilm communities. The biofilms formed in rich medium generally showed continuous growth over time; however, the absence of glucose resulted in significantly smaller biofilm volumes. A high proportion of viable cells (>90 %) was generally observed, and biofilm growth was correlated with high metabolic activity of cells. The community structure of biofilms formed in a rich medium did not change considerably over the 120-h period, during which E. faecalis, L. salivarius, and S. gordonii were most abundant.
A bovine tooth biofilm model system was developed by Lundström et al. [133] for the testing of different irrigation protocols. Permanent bovine incisors were coated with mucin and anaerobically inoculated with standardized suspensions of Streptococcus sanguinis, Actinomyces viscosus, Fusobacterium nucleatum, Peptostreptococcus micros, or Prevotella nigrescens. Teeth were randomly divided into four groups and rinsed for 3 min with 15 mL of irrigant. Biofilms were harvested and spiral-plated on selective media. The results provided strong evidence of a significant difference in the levels of bactericidal activity associated with the type of irrigant for all five bacterial species tested. Levels of antibacterial activity by NaOCl were significantly higher than by stabilized chlorine dioxide (ClO2) for S. sanguinis, A. viscosus, and P. nigrescens. The differences for F. nucleatum and P. micros were not significant.
Physiological Status of the Biofilm Bacteria
Biofilm bacteria are frequently encountered in challenging ecological environments in which they can best survive by activating various stress-responding mechanisms [67, 159]. A necrotic root canal represents a challenging environment in which bacteria face toxic substances such as bacteriocins and where they often have limited access to nutrients and certain key elements such as iron. This will force the bacteria to use various survival strategies such as reduced metabolic activity or in extreme situation transform into the “viable but non-culturable” (VBNC) state [157].
The physiological state of bacteria greatly affects the outcome of antimicrobial treatment. However, in most published studies, the biofilms have been grown for 1–7 days [37, 38, 160], while only occasionally have longer times up to several months been used [41, 43]. Few studies have compared the susceptibility of the biofilms to disinfecting agents at different stages of maturation. The importance of oral biofilm age and nutrition on biofilm behavior was recently demonstrated by Shen et al. [41], who exposed young and old biofilms (from 2 days to 12 weeks) to two different types of CHX preparations for 1, 3, or 10 min. The results of this study indicated that biofilms which were 2 weeks old and younger were much more sensitive to the antibacterial agents than biofilms grown for 3 weeks or more. It can be speculated that mature biofilms develop localized environments that dictate the metabolic activities of cells and better protect them against harmful effects of the environment. It must be recognized, however, that nutrients can produce changes within the environment of mature biofilms, such as variations in pH [161], so that the ability to survive or adapt to nutritional and other changes within mature biofilms remains an important aspect of the ecology of biofilm microbes. The results from this study [41] demonstrated that if only young biofilms of a few hours or even up to 2 weeks are used to assess the antibacterial efficacy of disinfecting agents, the results are likely to give a far too optimistic picture of their effectiveness. It is therefore important to understand the maturation curve of each biofilm model used and use mature biofilms when evaluating, e.g., the antibacterial efficacy of endodontic irrigants and other antibacterial materials.
New evidence of the effects of oral biofilm maturation on resistance to disinfecting agents was presented by Stojicic et al. [44], who, using the design described earlier [41], examined the effect of the source of biofilm bacteria, the level of biofilm maturation, and the type of disinfecting agent on the susceptibility of the biofilm bacteria to antibacterial agents. Multi-species biofilms from plaque bacteria of six donors were grown for up to 8 weeks on collagen-coated HA discs. After 1, 2, 3, 4, or 8 weeks of growth, the biofilms were exposed to 1 % NaOCl, 0.2 or 0.4 % iodine potassium iodide, or 2 % chlorhexidine for 1 or 3 min. The results showed that all 1- and 2-week-old biofilms were moderately or very sensitive to the tested disinfecting agents, which killed 20–99 % of the biofilm bacteria. After 3 weeks of growth, the biofilms became much more resistant to the same agents and only 10–30 % of the bacteria were killed using the same agents and exposure times. The same pattern of the effect of biofilm age (maturation) on the resistance of bacteria was observed in all six biofilms and with all three disinfecting agents. It is of interest that although the three disinfecting agents exert their antibacterial effect by different mechanisms, the development of biofilm resistance occurred similarly between 2 and 3 weeks of biofilm maturation for all three agents. The result emphasizes the importance of understanding the maturation timeline of each biofilm model which is used for testing the effectiveness of endodontic disinfecting agents against biofilm bacteria. So far, there has been little emphasis on this important aspect in the research on endodontic biofilms. With short biofilm maturation times, the results from these experiments will give too optimistic picture of the ability of the antibacterial agents to kill bacteria in the biofilms.
Persistent and recurrent apical periodontitis have been a focus of interest in endodontic research for a long time [161–165]. The primary cause of posttreatment apical periodontitis is acknowledged to be the continuing presence of bacteria within the root canal system [109, 166–169]. A histopathological investigation reported biofilm structures in the great majority (74 %) of cases of posttreatment apical periodontitis [168].
A variety of methods such as autoradiography; traditional colony count; 5-cyano-2,3-ditolyl-tetrazolium chloride (CTC); and LIVE/DEAD BacLight staining have been used to evaluate microbial viability. Traditional colony counting can only detect bacteria that are able to initiate cell division at a sufficient rate to form colonies and whose growth requirements are supported by the culture medium used. The bacteria can be sensitive to culture conditions (temperature, media, duration of incubation) [169]. The two-component BacLight staining has gained popularity because of its several potential advantages. It is a rapid and relatively easy-to-use test, and it yields both viable and total counts in one step. The two stains differ in their ability to penetrate normal and damaged bacterial cells. As a result, live bacteria with intact membranes fluoresce green (SYTO9), whereas dead bacteria fluoresce red, supposing that their membrane is damaged allowing penetration of the propidium iodine stain, which is responsible for the red fluorescence (Fig. 4.8). One recent study [157] examined cell culturability and viability using the two methods of bacterial detection in order to better understand bacterial behavior in a multi-species biofilm and to examine the possibility of the presence of the VBNC bacteria under long-lasting nutrient deprivation. The multi-species biofilm was grown from plaque bacteria on collagen-coated hydroxyapatite discs in BHI broth for 3 weeks (phase I) with a weekly addition of nutrients. This was followed by a 9-week nutrient-deprivation phase (phase II) with just one monthly addition of nutrients, after which the biofilm was reactivated again by weekly additions of fresh BHI medium for 4 weeks (phase III). The number and proportion of live bacteria in biofilm were assessed both by culturing and by confocal laser scanning microscopy using a LIVE/DEAD viability stain throughout the experiment. The results showed that the CFU counts dropped more than four logarithmic steps during phase II (nutrient deprivation), whereas the viability staining and confocal microscopy indicated only a 25 % drop in viability. Interestingly, the CFU counts started increasing during phase III when nutrient addition was changed back from once a month to once a week, but it took 4 weeks for the CFU counts to return (several logarithmic steps) close to the original CFU numbers. Cell viability, as indicated by the staining, improved from 75 % close to the original 95 %. The results strongly indicated that oral bacteria in a multi-species biofilm grown under nutrient deprivation remained viable but became unculturable. Interestingly, the bacteria could be recovered by renewed, more frequent access to fresh nutrients while still inside the biofilm. Viability staining thus seemed to better reflect the true viability of the biofilm bacteria than culturing during the long starvation phase. If this is the situation of in vivo biofilms in root canals with limited nutrition available to the bacteria, the results of this study may have an impact on the interpretation of results of cultural studies on root canal microbiology/biofilms in vivo.


Fig. 4.8
Three-dimensional constructions of confocal laser scanning microscope scans of 3-week-old multi-species biofilms after treatment with CHX-Plus® for 3 min. (a) Live bacteria (green); (b) dead bacteria (red); and (c) a combination of live and dead bacteria
Biofilms: Static Versus Dynamic
A number of different in vitro devices can be used to grow biofilms under continuous flow of fresh culture medium. Such in vitro devices are used to grow dynamic biofilms. The flow cell system is one of the most utilized in dynamic biofilm models. It has a transparent chamber of fixed depth through which the growth medium flows. The inlet tubing supplies growth medium and the outlet tubing drains the medium to a waste reservoir. The growth medium is passed through the cell with the aid of a peristaltic pump, which controls the flow rate of the medium. Prefabricated flow cell systems are available commercially or they can be custom-made based on any particular application. Fluid flow is considered to be a principal determinant of biofilm structure [170]. It provides nutrient exchange [171], influences density and strength [172, 173], and affects the dispersal of cells from the biofilm [174]. In a tooth with apical periodontitis, an exudate may move in and out of the root canal. This fluid exchange provides proteins, glycoproteins, and other nutrients to the bacteria growing as a biofilm in the root canal. However, despite the fluid/nutrient exchange, the flow rate is likely to be so low that it does not create shear forces that would have more than a minimal effect on the developing biofilms in the root canal. Therefore, it can be assumed that a static rather than dynamic biofilm model is a more realistic representation of the true situation of biofilms in the root canal.
The static model represents biofilms that have used up much of the available nutrients during growth and maturation. The key characteristics of such models are that numerous biofilms can be examined at any given time, and they can be used as a high-throughput system for biofilm analysis [175].
Inaccessible Root Canal Areas
Inaccessible regions of the root canal system (e.g., fins, accessory canals, and isthmi) cannot be examined by conventional microbiological sampling methods. The efficacy of passive ultrasonic irrigation at cleaning uninstrumentable recesses of the root canal system has been using artificially created grooves in both simulated root canals in plastic blocks [176, 177] and in extracted human teeth [178–180]. The grooves were packed with dentin debris followed by irrigation. Digital photographs were then taken and evaluated for the amount of residual debris. It should be emphasized though that these studies assessed the efficacy of the irrigation techniques on the visual cleanliness of the artificial grooves rather than the removal of bacteria, particularly those in biofilms.
Recently, Lin et al. [181] using extracted teeth with an artificial apical groove published a standardized biofilm model to quantify the efficacy of hand, rotary nickel–titanium and self-adjusting file (SAF) instrumentation in biofilm bacteria removal. Each tooth with an oblong canal was split longitudinally and a 0.2-mm-wide groove was placed in the apical 2–5 mm of the canal. After growing the polymicrobial biofilm inside the canal under anaerobic condition, the split halves were reassembled in a custom block, creating an apical vapor lock. Teeth were randomly divided into three treatment groups using a K-file, a conventional rotary NiTi file, or SAF. Irrigation was done using 10 mL of 3 % NaOCl and 4 mL 17 % EDTA. Areas inside and outside the groove were examined using SEM. Before treatment, a thick layer of biofilm was detected in the canals after 4 weeks of growth. Inside the groove, a smaller area remained occupied by bacteria after the use of SAF system rather than after the rotary file or hand K-file (3.25, 19.25, 26.98 %). For all groups, significantly more bacteria were removed outside the groove than inside, while no statistically significant differences were found outside the groove. The study demonstrated that none of the instrumentation techniques with irrigation was able to remove all bacteria from the studied area. This biofilm model represents a potentially useful tool for future studies of root canal cleaning in hard-to-reach areas.
Improved Models to Study Biofilms in Dentin Canals
Earlier approaches to establish the presence of bacteria in dentin canals have been based on culturing methods in which bacteria are grown in a liquid medium in the root canals of extracted teeth. Experience has shown, however, that only a low number of dentin canals are invaded by bacteria even after several weeks of incubation, and there are great variations from one area to another [99, 182, 183]. Producing comparable dentin infections with a predictable, heavy presence of bacteria has been difficult, making it challenging to determine the proportion of bacteria after exposure to various antibacterial irrigating solutions and other materials. A new dentin infection model was recently developed by producing a much more standardized infection deep in the dentin, by forcing E. faecalis into the dentinal tubules using a series of centrifugations at low and moderate speed [64, 184, 185] (Fig. 4.9). Before centrifugation, the opening of the dentin canals was enlarged by NaOCl and citric acid. Root surface cement was removed before the centrifugation to allow liquid (and bacterial) flow through the tubules. This dentin infection model not only provides a natural dentin canal environment for the bacteria to grow, but it also establishes a predictable presence of bacteria and model to quantitatively measure, using fluorescent viability staining and CLSM, the dynamics of bacterial killing after exposure to a variety of disinfecting agents. Negative controls with sterile water showed that E. faecalis survives the impact of centrifugation as the number of dead cells was similar to the number found in non-treated biofilms in which centrifugation was not used [184]. One of the limitations of these studies so far is that only a single-species biofilm model has been used instead of a polymicrobial biofilm model. On the other hand, E. faecalis is commonly found in persistent cases of endodontic infections, even in pure culture.
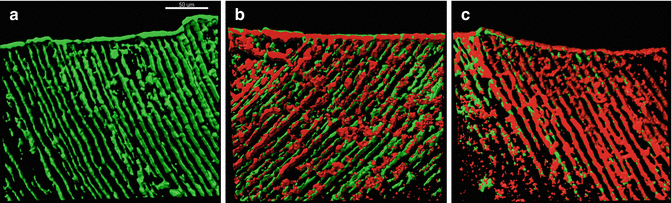

Fig. 4.9
Three-dimensional reconstructions of confocal laser scanning microscope images of E. faecalis-infected dentinal tubules treated by different concentrations of sodium hypochlorite (NaOCl) for 3 min, stained with viability staining. (a) Infected dentin treated with sterile water showing almost no dead bacteria; (b) dentin treated with 2 % NaOCl for 3 min shows moderate killing; and (c) dentin treated with 6 % NaOCl for 3 min shows high level of killing
Killing experiments using planktonic cultures often show differences of even several logarithmic steps between different medicaments or times of exposure. In biofilms, this is not the case, and typically the differences are within 10–50 % units only. Culturing, on the other hand, is not a sensitive enough method to reliably detect small differences in growth. The new dentin infection model with the high resolution of CLSM and viability stain makes it possible to detect significant differences even within the same logarithmic step, unlike in cultural studies of infected dentin. The percentage of killing of bacteria has been consistent from one study to another, and significant differences have been demonstrated between endodontic irrigation solutions and materials in these studies [64, 184, 185]. The studies have also demonstrated a great difference in sensitivity to disinfecting agents between young and mature biofilms in dentin canals [185]. The new standardized dentin infection model is a promising approach to study dentin disinfection not only by irrigating solutions but also by any material (sealers, cements, etc.) placed on the surface of infected dentin.
Dissolution of Organic Matter in the Root Canal
Sodium hypochlorite (NaOCl) is the most commonly used solution in endodontic irrigation because of its antimicrobial and tissue-dissolving activities. The ability of sodium hypochlorite to dissolve organic substances and thus to dissolve pulp fragments and debris is well known and documented. Tissues from a number of different sources have been used in studies assessing the tissue-dissolving ability of sodium hypochlorite [186]. Porcine muscle tissue [186–188], rabbit liver [189], rat connective tissue [190], pig palatal mucosa [191], bovine muscle tissue [192], bovine pulp [193], and pig pulp [194] have been used to determine the dissolution ability of different irrigants. There are a couple of methods to evaluate the dissolution in an in vitro study. One way is to measure the time of visualizing the end point of sample dissolution. However, it is difficult to determine the end point of complete dissolution of the tissue because of the large number of bubbles (resulting from the saponification reaction) attached to the sample surface. Therefore, fixed time has been used instead, and the samples have been weighed before and after exposure. Other methods have used different approaches, for example, measuring the changes in the solutions, such as the amount of available chlorine after completed dissolution [189] or the amount of hydroxyproline in the residual tissue after incubation with the solution [194].
The effectiveness of sodium hypochlorite relies on its concentration, volume, and contact time but also on the surface area of the exposed tissue [189]. High concentration NaOCl has a stronger effect, but it is also potentially more toxic to periapical tissue [195–197] in case of extrusion. Changes in dentin mechanical properties such as microhardness and roughness have also been reported after long-term exposure to sodium hypochlorite in concentrations of 2.5 and 5.25 % [198]. In one study [199] the authors reported that a 24-min exposure time to 2.5 % NaOCl caused a significant drop in flexural strength, while the modulus of elasticity was not affected during this time. Other authors found a decline of both flexural and elastic strength after a 2-h submersion of dentin bars in NaOCl [200]. The loss of calcium ions appears to be dependent on both the NaOCl concentration (5 % showing the greatest amount of decalcification) and the exposure time [201]. However, one of the shortcomings in models used in many of the studies of the effect on dentin properties by NaOCl and other solutions is that the natural anatomy/structure of dentin is often changed before the exposure. Dentin bars cut from the root dentin are usually devoid of the cement layer, thus allowing rapid penetration of the solutions through the entire thickness of the dentin pieces. In reality in the root canal, hypochlorite penetration into the surrounding root dentin is much more limited. Some studies have used powdered dentin which has been exposed to the irrigating solutions. The process of powdering may remove some of the hydroxyapatite protection around collagen fibers, possibly allowing more dramatic effects to occur. Therefore, new models where the structural integrity of the root dentin is preserved before the exposure are needed to secure a realistic understanding of the effects of endodontic irrigating solutions on dentin.
There are several ways to improve the efficacy of hypochlorite in tissue dissolution. These include increasing the pH [17] and the temperature of the solutions, ultrasonic activation, and prolonged working time [13]. Despite a general consensus that increased temperature enhances the effectiveness of hypochlorite solutions, relatively few articles have been published of the topic [20, 22, 202]. Preheating low-concentration solutions improves their tissue-dissolving capacity with no effect on their short-term stability. Also, systemic toxicity is lower compared with the higher-concentration solutions (at a lower temperature) with the same efficacy [22]. The impact of mechanical agitation of the hypochlorite solutions on tissue dissolution has been suggested to be important [188]. The study emphasized the great impact of violent fluid flow and shearing forces caused by ultrasound on the ability of hypochlorite to dissolve tissue [188]. However, the mechanisms involved are not completely understood [13]. Negative pressure irrigation was introduced to endodontic treatment several years ago as a safe method to effectively irrigate the most apical canals. Recently, a novel technology, the Multisonic Ultracleaning System (Sonendo Inc, Laguna Hills, CA), has been developed for cleaning of the root canal system. The system uses sound energy to create cavitation within the solution to remove soft tissue and bacteria inside root canals. Haapasalo et al. [203] compared the tissue-dissolving effectiveness of the Multisonic Ultracleaning System with conventional methods of irrigation using NaOCl in concentrations ranging from 0.5 to 6 % and at different temperatures (21 and 40 °C) of the irrigating solution. The results showed that the Multisonic Ultracleaning System demonstrated the by far fastest tissue dissolution. Tissue dissolution was more than eight times faster than the second fastest device tested, the Piezon Master 700 ultrasonic system. For all irrigation devices tested, the rate of tissue dissolution increased with a higher concentration and temperature of the NaOCl solution.
Sodium hypochlorite has a relatively low surface tension. Some investigators [204] have proposed adding a surfactant to sodium hypochlorite, in order to lower its surface tension and improve its ability to penetrate the principal canal, lateral canals, and tubules of dentin and predentin. The addition of surfactant would lower the surface tension by 15–20 %. The effect of the surface active agent to hypochlorite was first shown by Cameron [205] who demonstrated that the addition of the surface modifiers enhanced the ability of sodium hypochlorite to dissolve organic material. Clarkson et al. [186] tested the dissolution ability of three different brands of sodium hypochlorite available in Australia and reported that the products with surfactants dissolved porcine pulp in a shorter time than regular sodium hypochlorite at the same concentration. However, Jungbluth et al. [206] and Clarkson et al. [193] found no improvement in pulp tissue dissolution by NaOCl solutions containing surfactant compared with similar solutions without surfactant. The differences may be due to the study design and evaluation method. It should be noted that these investigations were all performed in the in vitro environment. Results may therefore not be directly extrapolated to the clinical situation. The active compound in NaOCl is the chlorine. NaOH-stabilized NaOCl has been suggested to have a stronger tissue-dissolving effect compared with the standard preparation [207]. The reason for this is that the OCl−/HOCl equilibrium adjusts itself exceedingly fast in non-stabilized solutions [207].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


