Introduction
Most composite resins release both bisphenol A (BPA), which disrupts the endocrine balance, and triethylene glycol dimethacrylate (TEGDMA), which has high risks for human health: eg, allergies and cytotoxicity. The aim of this study was to characterize monomers released from orthodontic adhesives.
Methods
We studied samples of orthodontic adhesives by associating 2 techniques: gas phase chromatography and mass spectrometry.
Results
The in-vitro analysis detected significant quantities of BPA, TEGDMA, and other monomers in orthodontic adhesives used in daily practice: Transbond XT, Transbond Supreme LV (both, 3M Unitek, Monrovia, Calif), Blugloo (Ormco, Orange, Calif), and MonoLok 2 (Rocky Mountain Orthodontics, Denver, Colo).
Conclusions
Clinicians should consider that orthodontic adhesives contain BPA, an endocrine disruptor; TEGDMA, an allergic and a cytotoxic compound; and carcinogenic genotoxic compounds. These molecules are not mentioned in the material safety data sheets. Manufacturers should declare all components of dental composites to identify these substances that may result in allergic or undesirable side effects for patients and dental staff.
Highlights
- •
Orthodontic adhesives contain fillers and resin matrix (bis-GMA, urethane).
- •
Fluidifiers such as triethylene glycol and polymers such as HEMA can be added.
- •
Gas phase chromatography-mass spectrometry measures monomers (types, quantities).
- •
Significant quantities of TEGDMA and bisphenol A have been detected.
- •
Manufacturers must declare components with potential undesirable side effects.
Bisphenol A (BPA) is a high-volume chemical, with over 6 billion pounds produced annually worldwide. This synthetic compound is found in epoxy resins and plastics used mostly in food packing and medical devices. Total adult intakes range from 30 to 70 ng per kilogram per day. According to the European Food Safety Authority, the tolerable daily limit for human exposure to BPA is 50 μg per kilogram. According to the Food and Drug Administration in the United States, the recommended daily limit is 5 mg of BPA per kilogram of weight.
Potential BPA sources in orthodontic materials are adhesives and polycarbonate esthetic brackets. The composite adhesive resins used in orthodontics consist of mineral fillers, resin matrix, generally BPA Bis-GMA (BPA diglycidyl-methacrylate) and urethane modified by the addition of fluidifiers belonging to the triethylene glycol family, catalysts (eg, camphroquinone), and various other additives. The self-etch bonding systems have introduced acid polymers and hydrophilic polymers such as hydroxyethyl-methacrylate (HEMA).
Elution of BPA may result from impurities left after resin synthesis, initially because of incomplete polymerization, and later because of resin degradation. BPA is an endocrine disruptor. It was first developed as a synthetic estrogen in the 1890s. Its effects are similar to those of natural hormones, and it blocks the cell hormone receptors. BPA may adversely affect humans (eg, reproduction, development, metabolic disease, inflammation, and gene expression). BPA also alters immune responses. Furthermore, BPA is rapidly metabolized by the liver into BPA β-D-glucuronide, which is also biologically active. Also, greater exposure to BPA bis-GMA–based dental composite restorations has been associated with impaired psychosocial function (anxiety, emotional symptoms, and clinical maladjustments) in children.
A review of the dental literature confirms the toxicity of the released monomers triethylene glycol dimethacrylate (TEGDMA) and HEMA, and indicates nonnegligible concentrations of residual BPA. Studies about the cytotoxic effects of orthodontic adhesives are limited. Gioka et al noted a reduction in DNA synthesis with orthodontic adhesive resins. Tang et al showed that chemical-cured paste-liquid adhesives were more cytotoxic than light-cured materials and chemical-cured paste-paste systems. Architectural and ultrastructural changes in epithelial cells because of the penetration of uncured primers were found by Vande Vannet and Hanssens. Goldberg found that TEGDMA and HEMA monomers are cytotoxic for gingival cells and are probably responsible for the allergies observed. BPA effects are observed at low doses, similar to concentrations in saliva or urine after dental composite placement.
Because these polymers are frequently used in dental therapy for children and adolescents, we thought it appropriate to assess the monomer release using an in-vitro model. Gas phase chromatography coupled with mass spectrometry was used to measure the types and quantities of monomers released by orthodontic adhesives and composite restoration resins.
Material and methods
Following the International Organization for Standardization (ISO) 10993-12:2012 standard for medical-device testing in biologic systems, samples 10 mm in diameter and 1 mm in thickness were prepared from orthodontic composites ( Table I ) and cured using a curing light (Elipar S10 LED; 3M ESPE, St Paul, Minn) with a power of 1200 mW per square centimeter and wavelength radiant energy between 430 and 480 nm for 20 seconds. The total surface area of a sample was 188.5 mm 2 . Each cured sample was immediately immersed in 0.4 mL (surface area/volume ratio = 3 cm 2 /mL ± 10%) of Milli-Q water (Merck Millipore, Billerica, Mass) in a glass tube for 24 hours at 37°C. The samples were then lyophilized, and 100 μL of dichloromethane was added.
| Product | Batch | Composition (material safety data sheet) |
|---|---|---|
| Transbond XT | N365309 | Bis-GMA: 10%-20% (CAS No. 1565-94-2); BPA bis 2-hydroxyethylether dimethacrylate: 5%-10% (CAS No. 24448-20-2); silane treated quartz: 70%-80% (CAS No. 100402-78-6); silane treated silica: <2% (CAS No. 68611-44-9); diphenyliodonium hexafluorophosphate: <0.2% (CAS No. 58109-40-3) |
| Transbond Supreme LV | N408552 | Bis-GMA: 10%-15% (CAS No. 1565-94-2); TEGDMA: 10%-15% (CAS No. 109-16-0); bis-EMA: 1%-5% (CAS No. 41637-38-1); silane treated ceramic: 52%-60% (CAS No. 444758-98-9); silane treated zirconium oxide: 3%-11%; silane treated silica: 3%-11% (CAS No. 248596-91-0); functionalized dimethacrylate polymer: 1%-5% |
| Blugloo | 4570074 | Glycidyl methacrylate: 3%-5% (CAS No. 106-91-2); inert fillers and pigments |
| MonoLok 2 light-activated bonding system | D2321-5 | Monomers of aromatic and aliphatic dimethacrylates; methacrylate monomers; camphorquinone; tertiary amine |
The composition of the eluates was analyzed using gas phase chromatography and mass spectrometry (TRACE Ultra Gas Chromatograph and ISQ Thermo; Thermo Finnigan Electron, Waltham, Mass). A capillary column 15 m in length, internal diameter of 0.25 mm, and film thickness of 0.25 μm was used with helium carrier gas at a flow rate of 1.5 mL per minute. The column temperature program was set as follows: initially, 50°C for 1 minute, increasing to 300°C at a rate of 15°C per minute. The temperature of the injector was 230°C, and the transfer line was 250°C. Mass spectra were obtained using electron impact ionization (70 eV, 150 μA, 200°C). The compounds primarily sought were BPA, TEGDMA, and HEMA.
Results
The chromatograms of each sample showed peaks according to their retention time for which the mass spectra, when compared with those of the National Institute of Standards and Technology database, correspond to chemical compounds.
The chromatograms are shown in Figures 1 through 4 .
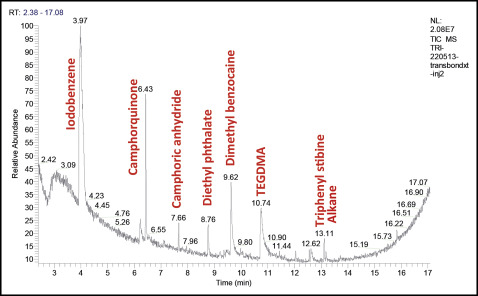
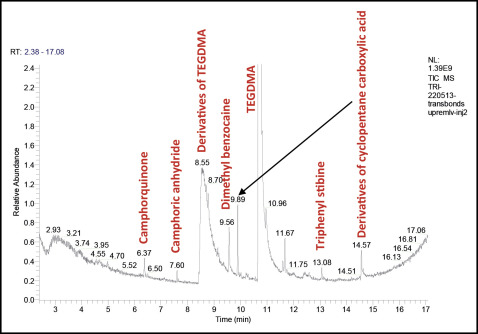
Many chemical molecules were identified in the commercial orthodontic adhesives tested by gas phase chromatography and mass spectrometry ( Table II ).
| Transbond XT | Transbond Supreme LV | Blugloo | MonoLok 2 | |
|---|---|---|---|---|
| Aromatic derivatives of trifluoroacetic acid C 2 HF 3 O 2 |
X | |||
| Iodobenzene C 6 H 5 I |
X | |||
| Camphorquinone C 10 H 14 O 2 |
X | X | X | X |
| Camphoric anhydride C 10 H 14 O 3 |
X | X | X | X |
| Butylhydroxytoluene C 15 H 24 O |
X | |||
| Derivatives of TEGDMA | X | |||
| Diethyl phthalate C 12 H 14 O 4 |
X | X | ||
| Dimethyl benzocaine C 11 H 15 NO 2 |
X | X | X | |
| Derivatives of cyclopentanecarboxylic acid | X | |||
| TEGDMA C 14 H 22 O 6 |
X | X | X | X |
| Acrylate derivatives | X | |||
| Tetraethylene glycol diacrylate and derivatives | X | |||
| Triphenyl stibine Sb(C 6 H 5 ) 3 |
X | X | X | |
| Aromatic derivatives dihydrobenzophenone types | X | |||
| BPA C 15 H 16 O 2 |
X | |||
| Alkane | X | X | ||
| Unidentified compounds | X | X |
Camphorquinone, camphoric anhydride, and TEGDMA were detected in all samples. Triphenyl stibine was found in 3 materials: Transbond XT, Transbond Supreme LV (both, 3M Unitek, Monrovia, Calif), and Blugloo (Ormco, Orange, Calif). Dimethyl benzocaine was detected in 3 products: Transbond XT, Transbond Supreme LV, and MonoLok 2 (Rocky Mountain Orthodontics, Denver, Colo). Diethyl phthalate was identified in Transbond XT and Blugloo. BPA was detected in only 1 material (Blugloo). Iodobenzene was detected in Transbond XT. Derivatives of TEGDMA and cyclopentanecarboxylic acid were identified in the composite Transbond Supreme LV. Butylhydrotoluene and tetraethylene glycol diacrylate and its compounds were found in Blugloo. Aromatic derivatives of trifluoroacetic acid, acrylate derivatives, and aromatic derivatives such as dihydrobenzophenone were identified in MonoLok 2. Of all these compounds, only camphorquinone was indicated on the MonoLok 2 safety data sheet; TEGDMA was indicated on the Transbond Supreme LV material safety data sheet ( Table I ).
Results
The chromatograms of each sample showed peaks according to their retention time for which the mass spectra, when compared with those of the National Institute of Standards and Technology database, correspond to chemical compounds.
The chromatograms are shown in Figures 1 through 4 .




