Fig. 10.1
Longitudinal section of the three mandibular molars (M1, M2, and M3). A cavity was prepared in the mesial part of M1. P pulp, C cavity
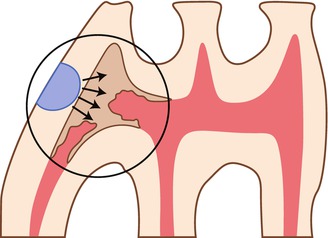
Fig. 10.2
Schematic drawing showing the formation of reactionary dentin beneath a cavity prepared in the mesial aspect of the first molar
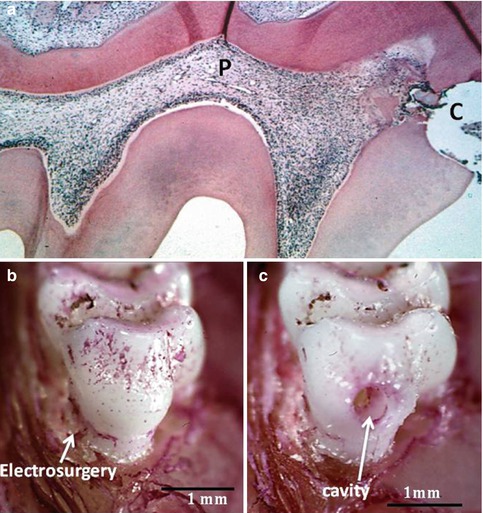
Fig. 10.3
(a) Histological section of the demineralized first mandibular molar. After the preparation of a cavity in the mesial aspect (c), a slight reaction occurs in the pulp (P). In (b), the tooth is seen after a cervical electrosurgery, and in (c), a cavity is drilled in the mesial aspect
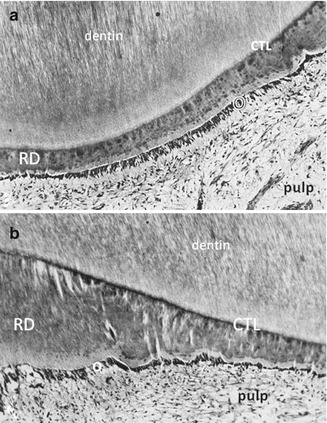
Fig. 10.4
Atubular reactionary dentin (RD) is formed by the odontoblasts and Hoehl’s cells, beneath a calciotraumatic line (CTL) at the junction between a tubular dentin and odontoblast layer (O). The dental pulp is fibrous but still alive. (a) Thin reactionary dentin formation; (b) thicker reparative dentin formation, produced in the same experimental conditions
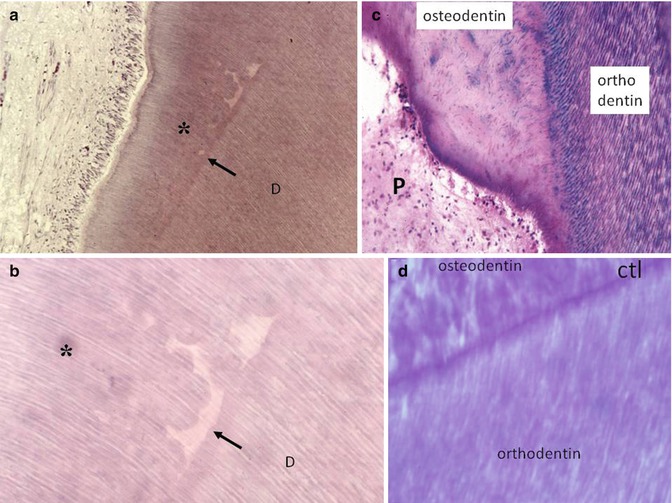
Fig. 10.5
(a, b) The preparation of a cavity induces the interruption of dentinogenesis. Beneath the tubular dentin formation, the dark arrows show interrupted physiological dentin deposit. The effects of the trauma lead to calcospherite formation (globular structures). The newly formed dentin (asterisks) comprises globular structures and interglobular spaces. Odontoblasts and pulp are “normal.” (c) After a more severe decay, tubular orthodentin (stained in purple by the “stains all” method) is interrupted; and the new osteodentin formed in reaction to the trauma, is atubular, and contains a few osteoblast lacunas. The dental pulp is apparently sound. (d) Reactionary osteodentin is separated from orthodentin by a calciotraumatic line (CTL)
If the carious lesion is progressing quickly, the lesion may destroy the residual dentin and penetrate the dental pulp. The pulp exposure favors the diffusion of bacteria from the contaminated dentin within the dental pulp. Pulp inflammatory cells control the infection and immune cells tend to slow down the progression of the lesion.
The reparative dentin–like structure that is formed constitutes an attempt to close the pulp exposure. In this case, odontoblasts and Hoehl’s cells are irreversibly wounded. Pulp progenitors or stem cells are implicated in the formation of a reparative dentin bridge or in a “bone-like” structure, also named osteodentin (Fig. 10.6a–d). Pulp cells are embedded in a bone-like structure similar to osteocyte-like cell being. These cells are located inside a lacuna. Reparative dentin-like fills partially or totally the mesial part of the pulp chamber of a rat’s molar. The dentin bridge seen at early stages of pulp capping expands and seals the mesial pulp chamber. The mineralization process expands up to the isthmus between the mesial and central parts. In humans, the reparative process is initially located beneath the pulp exposure. Then an osteodentin bridge gradually loads the pulp chamber. The mineralized structure is either in continuity with the newly formed reactionary dentin or appears as free pulp stone (calcospherites), developing around endothelial cells of capillaries.
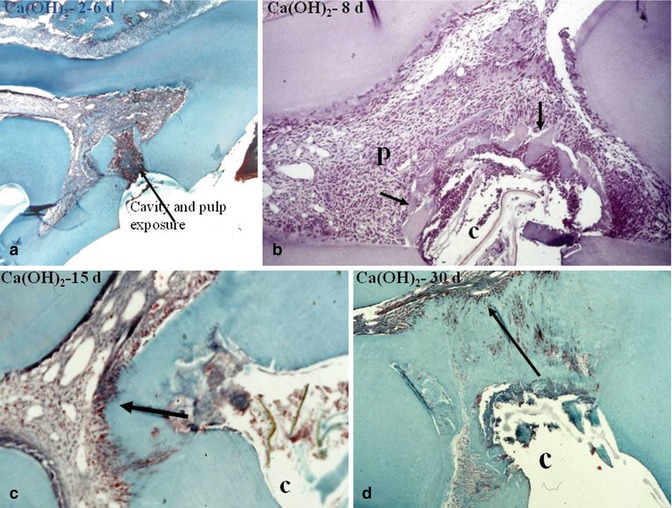

Fig. 10.6
In (a), 2–6 days after pulp capping a pulp exposure with Ca(OH)2, some dentin debris are pushed within the pulp. A pulp inflammatory process is limited to the mesial part of the pulp chamber. In (b), after 8 days, the calcium hydroxide induces the early formation of a dentin bridge, in close association with dentin debris (arrows). In (c), after 15 days, the dentin bridge is thicker and more homogeneous. (d) After 30 days, the dentin bridge occludes totally the pulp exposure. However, some tunnellike structures or cell remnants persist, allowing bacterial penetration toward the dental pulp. P pulp, C cavity
In many publications, some confusion occurs between reactionary and reparative dentin-like structures. To be precise, a clear-cut difference should be made between a tubular or atubular reactionary dentin, formed under the control of odontoblasts and Hoehl’s cells, and reparative dentin, appearing mostly as osteodentin and produced by pulp stem cells or progenitors.
A series of questions result from the available valid data. Wound healing results from repair or regeneration processes. Many major questions arise, including can we restore the original architecture and the biological function of the injured pulp tissue? Complete regeneration occurs during the fetal period, within the 24 weeks of gestation. However, in a clinical setting, we are treating young patients with already erupted teeth and/or adult patients. This implies that the postnatal wound healing combines a cascade of events leading to pulp repair and/or regeneration. The recruitment and differentiation of potential progenitors/stem cells are prerequisites. This is followed by pulp reconstruction.
10.2 In Vivo Approach
10.2.1 Preparation of a Cavity Without Pulp Exposure
In response to the preparation of a cavity in the mesial aspect of the first maxillary molar, odontoblasts, Hoehl’s cells, and pulp tissue reflect the reaction to the preparation of a cavity. Drilling a cavity caused the displacement of some odontoblasts and their penetration into dentin tubules. Inflammatory exudation was seen soon after drilling. The endothelium of capillaries showed an increase of pinocytic vesicles, an event associated with the formation of an exudative lesion. After 1 day, many damaged odontoblasts degenerate. Cells with a high nucleus/cytoplasm ratio and prominent nucleoli accumulate around the subodontoblastic capillaries. Newly differentiating odontoblasts received nutritional supply from the capillaries. After 3 days, differentiating odontoblasts increased in number. They start to produce reactionary dentin by 5 days after cavity preparation. Differences were seen between the original dentin layer formed before the drilling of the cavity and reactionary dentin-like formation [1].
10.2.2 Preparation of a Cavity Followed by a Pulp Exposure
Different repair responses were recorded between the coronal and radicular areas after the implantation of bone morphogenetic protein-7 (BMP-7) in a pulp exposure. Used as a capping agent, BMP-7 induces after 8 days the formation of a reparative osteodentin bridge, formed by globular mineralized areas at the exposure site and leaving unmineralized interglobular areas containing pulp remnants. A heterogeneous mineralization was seen in the coronal part of the pulp. Complete filling of the radicular pulp by a homogeneous mineralization was seen in the root, behind a calciotraumatic line. These results emphasize the biological differences between the coronal and radicular parts of the pulp [2]. Indeed, the crown is formed under the control of enamel organ, whereas the extracellular matrix (ECM) secreted by the epithelial Hertwig’s root sheath influences the root construction.
10.3 Reactionary Dentin (Tertiary Dentin)
Reviewing data on reactionary dentinogenesis, Smith et al. [3] differentiate between tertiary dentin and reparative dentinogenesis (Figs. 10.6a–d and 10.7a–d). It is indeed difficult to discriminate between the dentin secreted by postmitotic odontoblasts and a mineralized structure formed by a new population of pulp-derived, odontoblast-like cells. The tertiary dentin beneath a carious decay may comprise both reactionary and reparative dentins. Hence, a clear-cut definition for each of the tissues is needed and is difficult to obtain.
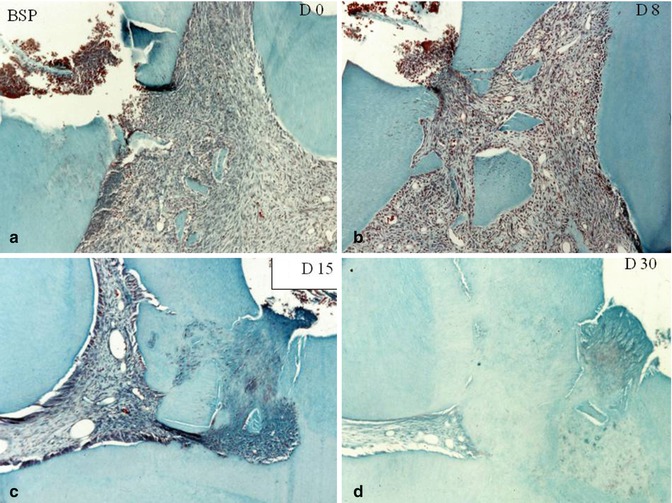

Fig. 10.7
Capping effects of bone sialoprotein (BSP). At day 0 (a) and at day 8 (b). Dentin debris are pushed within the pulp. (c) At day 15, beginning of the formation of a reparative dentin bridge. (d) 30 days after pulp capping with BSP, a thick and homogeneous dentin bridge closes totally the pulp exposure
Reactionary dentin is formed in response to a carious decay, to excessive abrasion, or to the cytotoxic effects of monomers released by a restorative material. This is how the pulp limits undesirable noxious effects. Trans-dentin stimulation is mediated by bioactive extracellular matrix components during a cavity preparation, the dental pulp being nonexposed. As an example, OP-1, used as a cavity liner, stimulates the formation of reactionary dentin [4]. Odontoblasts respond to the stimulating presence of ECM. Diffusion of ECM proteins, as determined by the residual dentin thickness (RDT) after preparation of a cavity, influences the signaling process [5]. They synthesize and secrete the ECM of a tubular or atubular dentin-like structure beneath a calciotraumatic line [6]. This line is similar to the reverse line seen in the bone. Sometimes this dentin-like structure is globular, but very often appears as lamellar or amorphous. Reactionary dentin also named tertiary dentin differs from the orthodentin formed prior to the lesion. The matrix of reactionary dentin displays reduced hardness and lowers elastic modulus. The residual dentin thickness (RDT) interferes with the pulp activity. The maximal dentin deposition of reactionary dentin appears beneath cavities with an RDT varying between 0.5 and 0.25 mm. Odontoblasts are reducing in number in cavities closer to the indirectly injured pulp. The restoration material influences also the odontoblast activities [7].
10.3.1 Molecules Expressed or Influencing Reactionary Dentin Formation
Differences were observed between reactionary dentin (RD) and primary or secondary orthodentin with respect to the distribution of five SIBLINGs (BSP, OPN, DMP–1, DSPP, and DSP (a fragment of DSPP)). BSP and OPN were observed in RD but not in predentin, whereas the expression of DMP-1 and DSP was lower in RD compared with predentin [8]. DSP was increased in density and spatial resolution. This molecule contributes to putative HAp nucleation on collagen scaffold. DSP antibodies showed weak staining in RD, whereas osteopontin (OPN) was extensively positive in RD [9]. OPN was not detected in physiological and reactionary dentin, but seen to be strongly immunoreactive in reparative dentin alone [10] (Fig. 10.7a–d).
10.3.2 Other ECM Molecules
-
Metalloproteinase–2 (MMP–2) is increased. Parallel upregulation occurs for TIMP-2 and for the membrane type 1-matrix metalloproteinase (MT1-MMP). Furthermore, the genes encoding components of the TGF-β signaling pathway, namely, SMAD–2 and SMAD–4, may explain the increased synthesis of collagen [11, 12].
-
Odontogenic ameloblast–associated protein (ODAM) is expressed by ameloblasts and odontoblasts. The molecule plays a role in enamel mineralization, possibly through the regulation of MMP-20. Experimental results show that rODAM accelerates reactionary dentin formation near the pulp exposure area, preserving normal odontoblasts in the remaining pulp [13].
-
Finally, the expression of Toll–like receptors was altered in response to caries.
10.4 Reparative Dentin-Like: From the Initial Formation of a dentin Bridge to Pulp Mineralization
10.4.1 Cells Implicated in Reparative Dentin-Like Formation
When odontoblasts are injured and destroyed, replacement cells express types I and III collagen gene-specific riboprobes. The cells forming reparative dentin synthesize type I collagen but not type III. Antibodies raised against DSP positively stain the cells. Therefore, they are odontoblast-like cells [14].
Adult human dental pulp stem cells (hDPSCs) have been isolated from the pulp as precursor cells. They differentiate into cells implicated in the formation of reparative dentin. Lysyl oxidase-like 2 (LOXL2) was downregulated when hDPSCs differentiate into odontoblast-like cells. Therefore, LOXL2 has negative effect on the differentiation of pulp cells into odontoblasts [15]. In contrast, the other LOX family members including LOX, LOXL1, LOXL3, and LOXL4 are increased.
Cells emerging from dental pulp explant were studied to elucidate the origin of precursor cells implicated in the formation of reparative dentin. Early outgrowing cells emerging from cultured explant were round or elongated, with thin spinous processes. They were highly mobile and contain numerous lipid vesicles. Radioautography suggested that these lipids resulted from micropinocytosis. After 10–20 days, the cells started to be converted into fibroblast-like cells, less mobile and lacking lipid vesicles. It was concluded that they might be mononuclear phagocytic/histiocytic mesenchymal cells [16].
In vivo, 10 days after Ca(OH)2 pulp capping, focal calcifications were seen within the collagen-rich matrix. Numerous extracellular matrix vesicles display electron-dense material composed of hydroxyapatite crystals [17]. At this early stage, similarities were detected between chondrocyte and the mineralization of cartilage septa and the early formation of reparative dentin.
Frozoni et al. [18] have used the 3.6-green fluorescent protein (GFP) transgenic mice to in vivo the biological sequence of events during pulp healing and reparative dentinogenesis. After pulp exposure and capping, followed by chemical fixation and processing for histological and epifluorescence analysis, immediately after pulp exposure no 3.6-GFP-labeled odontoblast was detected at the injury site. Four weeks after the surgery, reparative dentin started to be formed, and this was even more obvious at 8 weeks. The cells expressing 3.6-GFP lined an atubular dentin bridge. A few fluorescent cells were embedded within the atubular matrix, hence appeared as a bone-like structure (Figs. 10.8a–c and 10.9a–d).
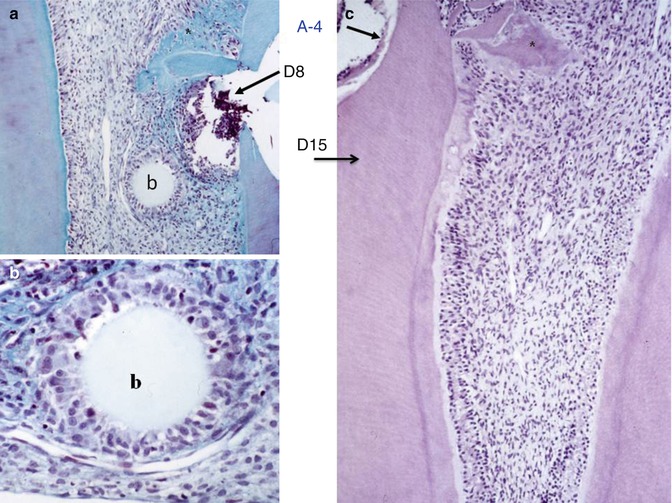
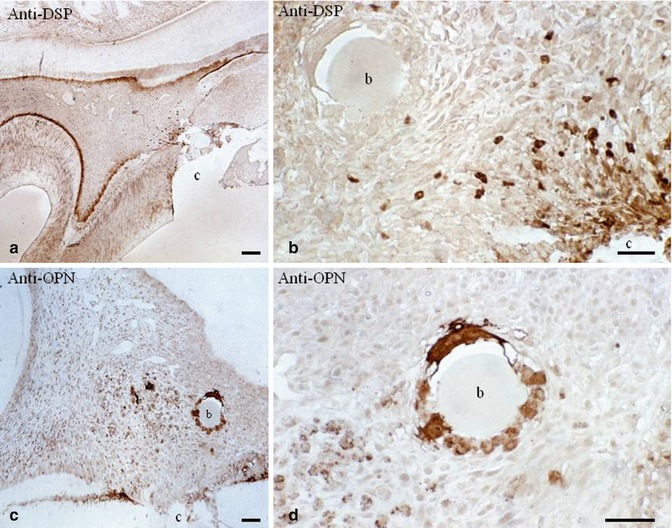

Fig. 10.8
(a) Implantation within the dental pulp of beads loaded with an amelogenin gene splice product A-4 after 8 days. (b) Committed cells are recruited and form a ring around the agarose bead (b). (c) At 15 days, reactionary dentin is formed uniformly along the root canal, behind a calciotraumatic line. In the crown, a mineralized area starts to be formed (asterisk)

Fig. 10.9
(a) After a pulp exposure and dentin sialoprotein (DSP) immunolabeling, an odontoblast layer is densely stained. (b) No staining is detected around the beads (b), but near the pulp exposure (c cavity), odontoblast-like cells are well stained by the antibody. (c) In contrast, osteopontin immunolabeling is dense around the carrier bead (b) and in close proximity. (d) The cells located around the bead are densely immunostained by the anti-osteopontin. The two immunostainings reveal that the nature of cells nearby the pulp exposure (DSP dentin ECM protein) is very different from the cells implicated in pulp inflammation and bone formation (osteopontin)
Dentin regeneration may be obtained by using porcine deciduous pulp stem cells mixed with β-tricalcium phosphate. Four months after transplantation, regeneration of a dentin-like structure was completely completed, closing the roof defects [19] (Fig. 10.10a, b).
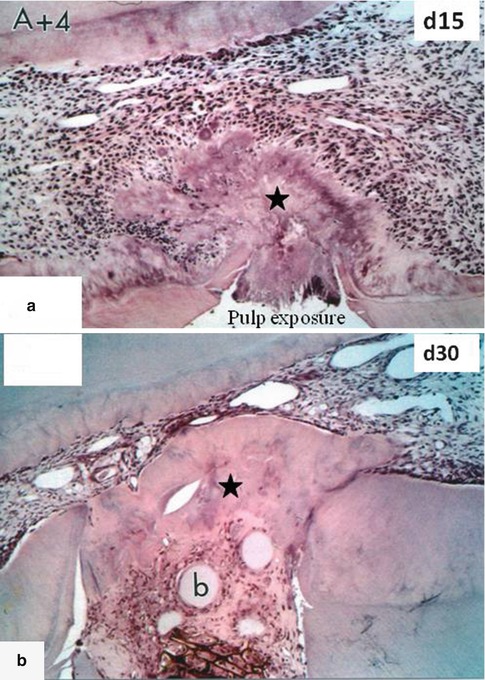

Fig. 10.10
In (a), A+4 was implanted in the dental pulp 15 days earlier. A forming homogeneous reparative dentin bridge (asterisk) is closing the pulp exposure. (b) After 30 days, the reparative dentin bridge is much thicker (asterisk) and many beads (b) are embedded within dentin
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


