Fig. 9.1
Macroscopical views of progressive stages of caries in different molars. (a) Occlusal lesion without clinical dentine exposure in mandibular molar. (b) Occlusal lesion with initial dentine exposure reflecting a closed lesion environment in a mandibular molar. (c) Occlusal lesion with established dentine exposure. Note that the undermined enamel is reflected as change in enamel translucency surrounding the clinical cavity, also described as retrograde demineralization along the enamel-dentine junction. A macroscopical cutting plane of this lesion is shown in Fig. 9.2b. (d) A gradual breakdown is taking place in a mandibular molar; note the milky appearance of the cavity border reflecting the retrograde demineralization. (e) In principal, a more advanced stage of enamel breakdown from a maxillary molar. The ecosystem has started to become more open, and the change in microbial growth condition is clearly reflected by a less pronounced biofilm. (f) The occlusal enamel here is completely broken away and a “new” occlusal dentine surface has emerged. The clinical appearance of the carious dentine is dark at the central area. This case represent a clinical example of an open lesion environment, with a temporarily arrest at the occlusal site, whereas along the peripheral borders the cariogenic biofilm is clearly present, maintaining a high lesion activity. The case reflects the relative importance of a marked change within a local ecosystem
The diagnosis of the condition of the pulp has been systematically reviewed [6] and with only a few papers, accepted for inclusion. Of these, the scientific level of evidence was most often low due to methodological shortcomings. However, the following features were found:
-
No obvious association between cold, heat, electric pulp test, and percussion in deep asymptomatic caries and status of pulp inflammation [9].
-
Irrespective of the degree of inflammation, the majority of patients may respond to a percussion test, even though the teeth have minimal or no pulp inflammation.
-
It is important to stress the unreliability of pain as a parameter for the clinical assessment of reversibility/irreversibility of pulpal inflammation. Severe inflammatory reactions can be observed in teeth with no history of pain.
-
Despite the presence of spontaneous pain, indicative of irreversible pulp inflammation, histologically it is possible to find no evidence of pulp exposure or necrosis in the pulp tissue, indicating that the point of irreversibility had not been reached.
-
Absence of pain does not exclude the presence of inflammation.
It has recently been addressed [10] that some of the study shortcomings may relate to the fact that the term “deep lesion” has been used without a more detailed definition of the actual depth of the lesion per se. Was it deep dentine involvement? Is it based on x-rays? Is caries in contact with the pulp clinically, radiographically, or by means of subjective symptoms indicating pain, or is it measured in terms of histology? If this information is missing, it is difficult from a clinical aspect to interpret anything about the pulp.
9.3 Previous Histological Shortcomings Have Simplified the Understanding of Caries Pathology
It should not be underestimated that almost all previous histological studies of the pulp have been based on demineralized histological sections in order to be able to cut thin sections. However, during the decalcification procedure of the tooth specimen, not only the mineral content of the dentine but also the entire enamel is removed. Consequently, important information about the lesion environment has therefore been lost. This may have led to several examples of oversimplification in the understanding of caries pathology:
-
Studies attempting to correlate enamel caries with pulpal inflammation have been described as being speculative [11].
-
The early spread of caries into the dentine was believed to be the same as seen in advanced stages of lesion progress with signs of huge dentine exposure and undermining enamel [12].
-
Teeth with rapidly progressing caries have dominated the materials investigated, presumably because they were the ones available for extraction [6].
Consequently, it was taught that even a small enamel lesion without histological contact to the enamel-dentine junction could induce both the translucent zone and the demineralized zone in the dentine [13] and in particular the non-cavitated enamel lesion could hide bacterial invasion [14] as well as undermine sound enamel from the very beginning, even without visible dentine exposure [12–17]. Therefore, many dentists of the last century were trained to both remove the early enamel lesion by operative intervention, as well as to be radical when performing dentine excavation, because carious dentine left over was believed to maintain the pulp inflammation and eventually lead to pulp degradation. The description of the changes in the pulp has often been closely related to scenarios of “points of no returns” in terms of inflammation. Clinically, the so-called deep caries progression should therefore not be treated with an indirect pulp capping [4, 11], as the retained carious tissue would maintain further development of pulp inflammation. In contrast, complete excavation was recommended even if it led to exposure of the pulp, because the pulp was judged to be irreversibly inflamed anyway, even though the patient was not in pain. Moreover, if already an enamel lesion is able to induce inflammation, it would be easy to imagine that a deep lesion would be associated with unwanted inflammation.
However, the advances of pulp biology [18–23] combined with at detailed description of the lesion environment, as well as the clinical evidence of treating deep lesions [24–28], have started to modify the traditional view of pulp inflammation as being “the nonstop train” toward apoptosis and pulp necrosis.
9.4 Progression of the Non-cavitated and Cavitated Enamel-Dentinal Lesion Complex
A brief update of principal caries pathology is presented. Detailed histomorphological studies have revealed that the carious enamel-dentine lesion complex is a closed entity as long as there is no destruction and disintegration of the demineralized enamel [8]. The cariogenic biomass is located at the enamel surface only. The enamel lesion contact with the enamel-dentine junction is strictly related to the extent of the demineralized dentine, and no early spread along the enamel-dentine junction can be monitored (Fig. 9.2a). When the established caries remain untreated, they advance in width and depth. The dentine is clinically exposed (Fig. 9.1c, d) and the biofilm is heavily involving the cavity. The microbial ecosystem can be described as being a “closed” environment. At this stage the spread along the enamel-dentine junction is a characteristic feature undermining sound enamel [29] and the lesion becomes wedge shaped, with the base directed toward the surface (Fig. 9.2b). As the deep lesion further progresses, the undermined enamel often breaks off (Fig. 9.1d–f), due to masticatory forces, converting the lesion environment from a “closed” toward a more “open” lesion environment [30]. Clinically and macroscopically the color of the demineralized dentine becomes darker (Fig. 9.2c), and at the central area the cariogenic biofilms overlying the lesion are markedly reduced. Although the lesion has increased in size, the actual activity has temporarily decreased. Even at the macroscopic level, progressive alterations of pulp inflammation can be observed in terms of increasing visualization of the vascular architecture in the pulp (Fig. 9.2a–c).
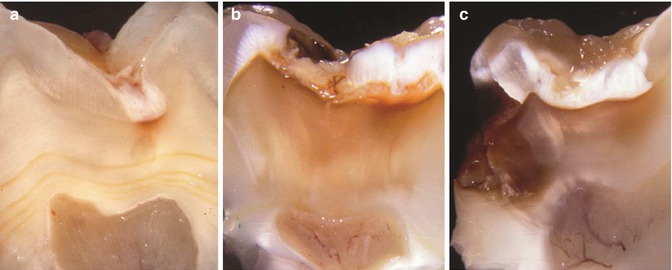

Fig. 9.2
Macroscopical cutting profiles in relation to progressive stages of caries. (a) An occlusal lesion without dentine exposure. A close interrelation is noted between the enamel lesion contact and the extent of the affected dentine; no visible alterations in the pulp. (b) Macroscopical cutting profile of lesion shown in Fig. 9.1c. Note the retrograde demineralization along the enamel-dentine junction as well as the initial appearance of the pulpal vascular architecture. (c) The cutting profile of a mixed lesion environment. Note a more marked appearance of the vascular architecture. From [10] with permission from Elsevier
9.5 Carious Enamel-Dentine Lesion Complex and Activity: Understanding the Initial Pulp Response Subjacent to Enamel Lesion Without Cavitation
Studies have shown that the cytoplasm/nucleus ratio of the mature odontoblast cells, subjacent the superficial enamel lesion, is more reduced than unaffected control sites, even before visible alteration in dentine mineralization. Moreover, the subodontoblastic or Höehl’s layer appears indistinct [26]. Enhanced mineralization of the dentine is noted in the central and oldest part of the involved lesion area, as the enamel lesion progresses toward the enamel-dentine junction. At the pulpal site substantial growth of the predentine matrix is noted with bundles of collagen fibers aligned with odontoblasts reduced in size [8]. These first findings of cellular alterations and enhanced mineralization of the dentine are probably not associated with antigen-related pulp reactions as the bacterial-induced dentine demineralization is not yet established.
9.6 The Trigger of Pulpal Immunity in Progressive Stages of Carious Dentine
It is well known that the dentine comprises bioactive extracellular matrix [31–35], and during carious demineralization of the dentine, there is a release of bioactive molecules [22, 36, 37], which can trigger the odontoblasts, members of the first line of host defense (see Chap. 7). However, it is unclear whether this may take place even before bacteria have invaded the demineralized dentine (Fig. 9.2a), also defined as affected dentine [38]. In non-cavitated enamel lesions with subjacent dentine demineralization in humans, observations of a reduced odontoblast layer, as well as the alterations in the subodontoblastic region, may qualitatively reflect an early odontoblast-triggered innate immune response [18]. Moreover, a different pulp response is noted subjacent an active versus a slowly progressing lesion. The cytoplasm/nucleus ratio of the odontoblasts appears markedly reduced in both scenarios, but a maintained indistinct subodontoblastic region is noted only in active sites [31].
In a series of in vitro studies, the odontoblast-like cells were shown to express Toll-like receptors [39–42] making the cells capable to induce mediators known to influence positively or negatively both the inflammatory as well as the immune responses in pathogen-challenged pulp-like tissue. As an example lipoteichoic acid, which is a by-product from Gram-positive bacteria, was able to elicit proinflammatory cytokines by further promoting immature dendritic cell recruitment [42].
The Gram-positive bacteria represent the first members of invading bacteria in demineralized dentine with a clinical dentine exposure (Fig. 9.2a), eventually accounting for 70 % of the cultivable microbiota in lesion sizes involving two-thirds of the dentine and more [43]. During the development of such a “closed” ecosystem, a remarkable homogeneous lactobacilli microflora can be detected. Gram-negative bacteria take over [44] as the lesion advances, and, because of their content of lipopolysaccharide, they are capable of inducing lipopolysaccharide–binding protein which has an even more complex role in terms of triggering the odontoblast-like cells. Recently, it was found that this protein may interact with lipoteichoic acid, hence reducing the receptor-dependent production of inflammatory cytokines by odontoblast-like cells and in this way modulate host defense in human dental pulp [45]. The various laboratory setups [18–22, 32–37, 39–42] with, for example, the odontoblast-like cells have helped gain more and more complex information, eventually leading to an improved understanding of the host defense as well as of tooth repair in the future. However, we are not there yet, in terms of the application of knowledge transformed into treatment modalities in humans.
9.7 The Dentinal Lesion with Clinical Exposure: The Infected Dentine
The established caries lesion with a visible dentine exposure (Fig. 9.1b, c) will comprise all the classical zones of carious dentine: the zone of sclerotic dentine (i.e., increased intratubular mineralization), the dentine demineralization, and, finally, the zone of bacterial invasion and dentine degradation [8]. When the bacteria invade demineralized dentine and it becomes infected [38], the vast majority of the members of the biofilm are Gram-positive bacteria in primary dentine and the innate immune systems are progressively activated. It is not known how the triggered dental pulp immunity is operating during a slow lesion progression. As tested during stepwise excavation of dentin caries, the cultivable microflora becomes markedly reduced in numbers [46], consequently the acidogenic pH levels decrease, which presumably also leads to decreased production of, for example, lipoteichoic acid. Finally, this may temporarily stop the sequence of events leading to a further unwanted inflammatory response. In confirming this, the tertiary dentine appears like a continuous formation of secondary dentine laid down along a slow lesion progression, whereas the tertiary dentine appears partly atubular during ongoing stages of active lesion progress (Fig. 9.3).
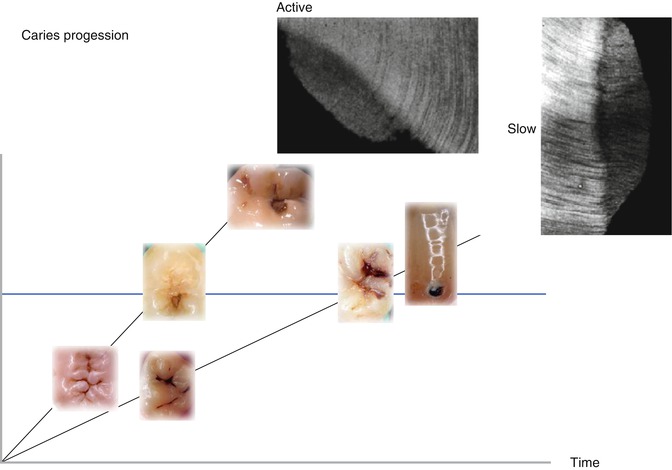

Fig. 9.3
A principal demonstration is shown of an active versus a slow lesion progression. Typically the texture of the tertiary dentine is different within the two scenarios as shown by inserts of two microradiographs of undemineralized thin sections. Along the x-axis, the principal caries progression is shown by inserts of clinical illustrations of progressing lesions. The y-axis represents the time line. The different slope of the two scenarios reflects the different speed of progression
Taken together, the odontoblasts are members of the first line of defense responding to various carious stages and activities. How this modulates the role of the odontoblasts during early tertiary dentine formation in humans remains unclear, but it is detailed in Chap. 10.
9.8 The Definition of Deep and Extreme Deep Carious Lesions
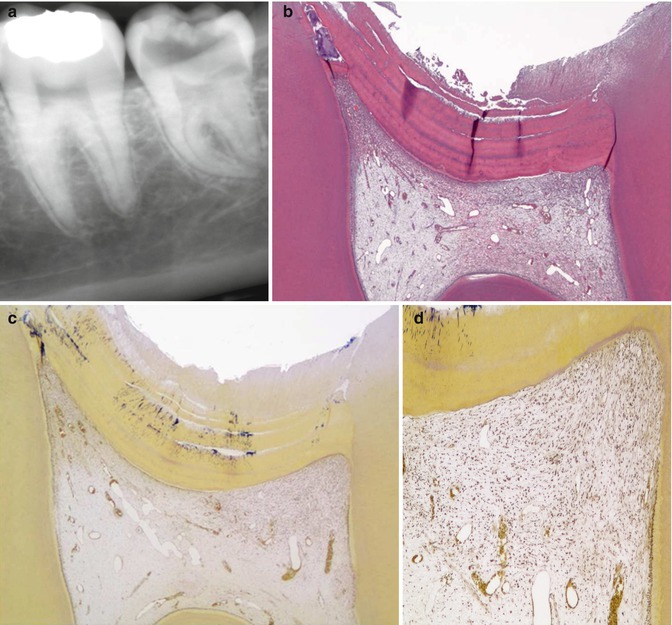
A definition of deep caries has to be made by the x-ray, because it is within the clinical setting that the general dental practitioners will end up using the findings of laboratory research, and therefore it is of paramount importance to have a reference that can be used in a clinical setting. As the depth of the caries lesion represents a diagnostic problem, it may be relevant to further classify it beyond previous attempts. The deep lesion has previously been defined radiographically as being within the pulpal quarter toward actual contact with the pulp [47]. However, we suggest separating the deep lesion in two scenarios. A deep caries lesion is defined as involving the pulpal quarter of the primary/secondary dentine (Figs. 9.4b. 9.5a, and 9.6a) but still with a radiodense zone separating the demineralized dentine [19]. The extreme deep lesion involves the entire primary/secondary dentine either with no radiodense zone separating the demineralized dentine from the pulp or with a radiodense zone located within the pulp chamber indicative of tertiary dentine (Figs. 9.4a, 9.7a, and 9.8a).
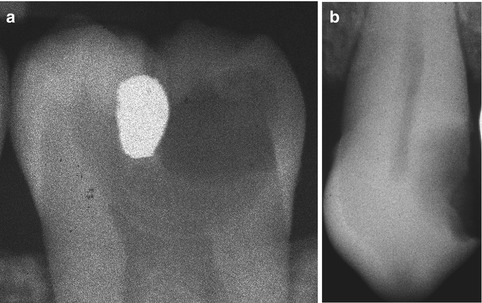
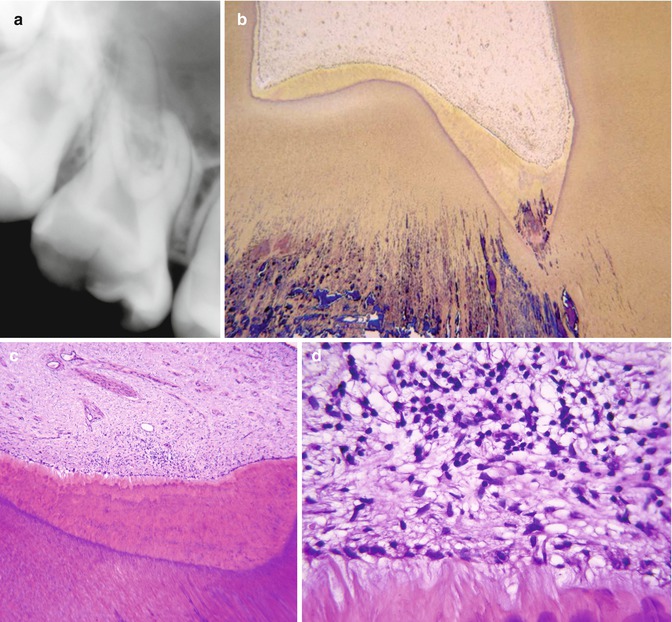
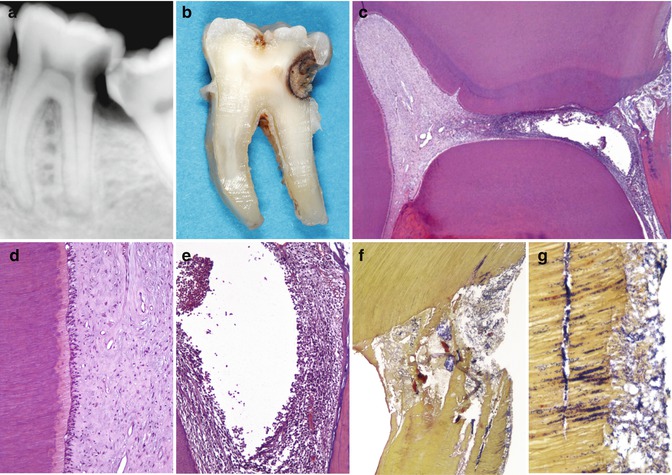

Fig. 9.4
The radiographic presentation of an extreme deep caries lesion versus a deep lesion. (a) The entire primary/secondary dentine is penetrated either with no radiodense zone separating the demineralized dentine from the pulp or with a radiodense zone located within the pulp chamber indicative of tertiary dentine. (b) The deep lesion involves the pulpal quarter with a radiodense zone separating the translucent zone from the pulp

Fig. 9.5
Deep caries is shown with reversible pulp inflammation. (a) The caries lesion shown in Fig. 9.1e with a more advanced stage of enamel breakdown due to the undermining nature of caries progression. The patient complained of pain to thermal stimuli and chewing. Sensitivity tests gave exaggerated responses. There is no apical periodontitis lesion. (b) Overview encompassing the caries lesion, tertiary dentine and pulp. Note how the tertiary dentine is heavily infiltrated by bacteria in the pulp horn area. No necrosis could be observed in this and in any of the serial sections. The histological diagnosis is “reversible pulp inflammation” (Taylor’s modified Brown and Brenn, orig. mag. ×25). (c) Considerable amount of tertiary dentine is formed on the pulpal side (H&E, orig. mag. ×50). (d) The tertiary dentine is covered by an incomplete layer of flattened odontoblasts. Subjacent to it, a severe concentration of chronic inflammatory cells is present (orig. mag. ×400)

Fig. 9.6
Deep caries is shown with irreversible pulp inflammation. (a) Mandibular second molar in a 22-year-old man. Severe spontaneous pain. The radiograph shows a deep distal caries proximal to the pulp. At the distal root there is an indication of an apical periodontitis lesion. The patient did not accept any treatment aimed at conservation of the tooth and requested extraction. (b) Photograph taken after grinding the tooth on a mesiodistal plane until the pulp was seen. (c) Overview of the pulp chamber. A proximal deep lesion is noted distally. The distal half of the pulp chamber tissue is necrotic, while the mesial portion exhibits a relative normality (H&E, orig. mag. ×16). (d) Detail of the left wall of the mesial pulp horn in c. The pulp shows normal histological features (orig. mag. ×100). (e) Detail of the distal root canal orifice area in c. Liquefaction necrosis surrounded by severe concentration of inflammatory cells (orig. mag. ×100). (f) Bacterial penetration in the distal pulp horn area (Taylor’s modified B&B, orig. mag. ×50). (g) High power view of the caries lesion. Dentine is heavily colonized by bacteria. A distinct bacterial biofilm is present on the cavity floor (orig. mag. ×400)
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses