Fig. 1.1
At the initial stage of tooth germ formation, a basement membrane (BM) separates the outer epithelium (epithelial placode) (OE) from the subjacent mesenchyme. The future pulp (P) (condensation zone) displays type IV collagen immunostaining limiting capillaries (cp)
The first evidence of the future teeth appears when the epithelial cells near the basement membrane begin to multiply (four to five cell layers) and invaginate into the underlying ectomesenchyme, giving rise to the dental lamina. The ectomesenchyme starts to change composition in response and becomes more condensed. Thus, this initial epithelial invagination clearly marks the apparition of the tooth crown area and will develop through several distinct stages (bud, cap, bell stage). Tooth development is a continuous process, so clear distinction between the transition stages is not possible.
Each dental lamina is at the origin of a tooth bud (Fig. 1.2). Tooth buds of the deciduous canines and incisors are apparent in the 8-week-old human embryo, and buds of the deciduous molars are formed during the ninth week. The bud stage is characterized by the progression of ectodermal invagination in the underlying ectomesenchyme, in which cells are packed closely around the epithelial bud. This will be followed by the changes in the shape of the dental bud and formation of the dental cap (Figs. 1.2 and 1.3). The cap stage is characterized by a concavity of the epithelium that partially envelops the underlying mesenchyme. During the cap stage, the epithelial outgrowth is referred widely as the enamel organ and is related to the differentiation of the outer dental epithelium, inner dental epithelium, and the appearance of the enamel knot. Also, there is a condensation of the ectomesenchyme in the concavity of the enamel organ forming the dental papilla, at the origin of the odontoblasts and the dental pulp [51, 52] (Figs. 1.4 and 1.5).
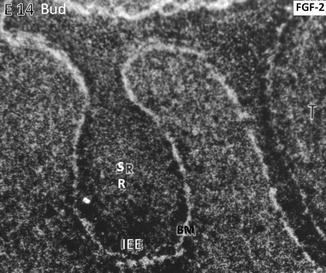
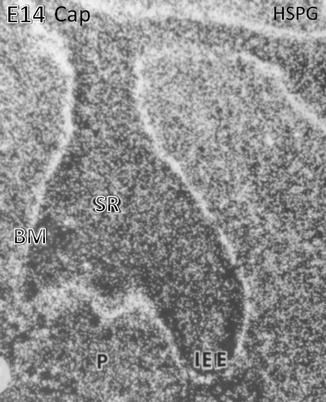
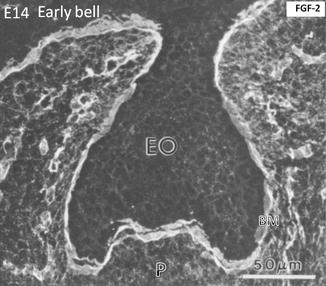
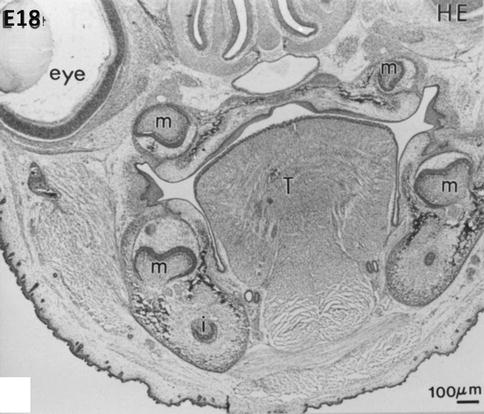

Fig. 1.2
At the embryonic day, E14 binding of the radioactive FGF-2 occurs along the basement membrane (BM) limiting the inner epithelial epithelium (IEE). The central part of the tooth bud contains the stratum reticulum (SR)

Fig. 1.3
Heparan sulfate proteoglycan (HSPG) binding is mostly situated along the basement membrane (BM) located at the early cap stage along the inner enamel epithelium (IEE) above the stratum reticulum (SR). In the embryonic pulp (P), numerous cells are immunostained

Fig. 1.4
At a more advanced cap stage (day 14), dense FGF-2 binding occurs along the basement membrane (BM). The binding is weaker in the enamel organ (EO), limited to the cell surface. The binding is stronger in the pulp (p), capillaries, and the early stages of the trabecular bone

Fig. 1.5
At embryonic day 18, hematoxylin-eosin staining reveals early bell stages of molars (m) in the mandible (lower part of the figure) and maxillary (upper part, near the nasal cavities, the mineralizing palatal layer, and the eye). The tongue (T) occupies the central part of the mouse head. Beneath the molars, the incisors are seen in the transverse sections of the mandible
Starting from the late cap stage and through the transition from cap to bell stage of tooth development, many developmental changes are observed. All the elements of the enamel organ are well distinguished (histodifferentiation):
-
The outer enamel epithelium located at the periphery of the cap in contact with the peridental mesenchyme.
-
The inner enamel epithelium formed by cells which are precursors of ameloblasts and which are separated from the future dental pulp by a basement membrane.
-
The stellate reticulum and the stratum intermedium, two intermediate layers of the enamel organ involved in the transcellular and intercellular transfer of precursors of enamel proteins, and in the provision of energy for these transfers (synthesis and degradation of glycogen). For some authors, the stratum intermedium differentiates during the bell stage.
-
The primary enamel knot, a transient structure situated in the center of the enamel organ that will control the morphogenesis of dental cusps and determine the final shape of the tooth [53]. Subsequently, secondary enamel knots will be formed, contributing to the formation of molar cusps. The enamel knots within epithelium are described as organizing centers composed by “clusters” of cells that secrete many morphogen signals like Shh, Wnts, FGFs, and BMPs, whose roles are not yet fully defined.
Besides the role of the enamel knots to regulate the size and shape of the teeth, the signals from the mesenchyme are also necessary for the formation and maintenance of epithelial compartments.
The cap stage is followed by the bell stage, during which the dental crown acquires its final shape (morphodifferentiation) and the formation of the cusp pattern is observed (Figs. 1.6 and 1.7).
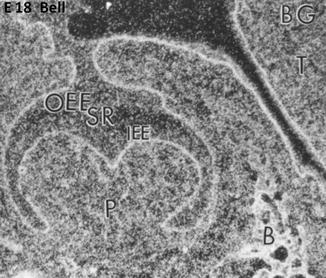
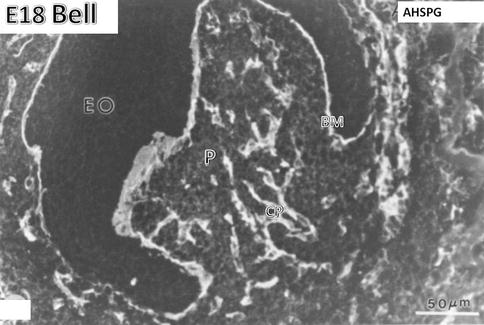

Fig. 1.6
E18. At the bell stage, the binding of FGF-2 (BG) concentrate along the basement membrane in the outer enamel epithelium (OEE), stellate reticulum (SR), and inner enamel epithelium (IEE). The pulp (P) is heavily labeled and the forming bone (B) as well. T tongue

Fig. 1.7
E18. Bell stage. Anti-heparan sulfate proteoglycan (AHSPG) immunolabeling. No labeling is detectable in the enamel organ (EO). The basement membrane (BM) is densely immunostained. In the pulp (P), the capillaries (CP) are well stained. At the stage of crown formation, endothelial cells of capillaries proliferate, elongate, and form a dense vascular network
The outer and inner enamel epithelia are continuous, and they meet at the rim of the enamel organ known as the zone of reflection or cervical loop. Extended in Hertwig’s epithelial root sheath, this cervical loop will control the formation of the root, including the root odontoblast differentiation (Figs. 1.8 and 1.9). The cervical loop progresses in apical direction, and many cell divisions sustain this preeruptive crown growth, thus delimiting increasingly the dental papilla area. In the pluricuspid teeth, the secondary enamel knots appear at the top of each cusp.
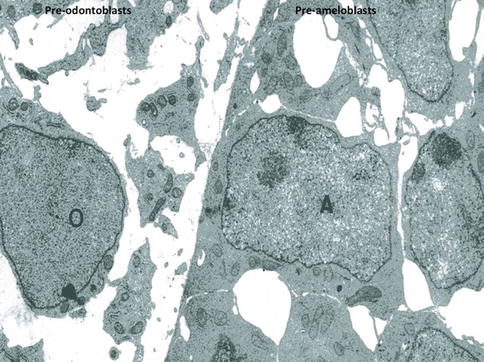
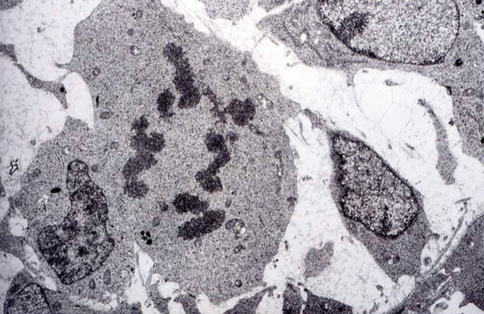

Fig. 1.8
At a later stage, pre-odontoblasts are facing pre-ameloblasts. Postmitotic polarizing ameloblasts form a cell network, with large intercellular spaces. Presecretory pre-polarized odontoblasts (O) are located in an electron-lucent dental pulp

Fig. 1.9
The ultimate asymmetric division allows pre-odontoblasts to become postmitotic odontoblasts implicated in dentin formation
In the late bell stage, tooth morphogenesis is followed by a phase of cell differentiation of the inner enamel epithelium and of the ectomesenchymal cells at the epithelial-mesenchymal interface with the basement membrane (histodifferentiation). These cells will differentiate in pre-ameloblasts and pre-odontoblasts in order to become polarized and secreting ameloblasts and odontoblasts to form the enamel and dentin, respectively. The first layers of the enamel and dentine are visible at the end of the coronary morphogenesis. Thus, during embryogenesis, morphogenesis and differentiation are coupled. Cells acquire the competence to differentiate according to their position. Differentiation of pre-ameloblasts and odontoblasts is pre-coupled spatiotemporally to these morphogenetic movements that provide the pattern formation of the crown and the beginning of the root formation.
The condensed ectomesenchyme situated at the periphery of the enamel organ and dental papilla is referred as the dental follicle or dental sac and will give rise to the supporting dental tissues as the tooth cementum, periodontal ligament, and alveolar bone. Thus, the dental follicle is involved in the formation of the root and tooth eruption (Figs. 1.5 and 1.10).
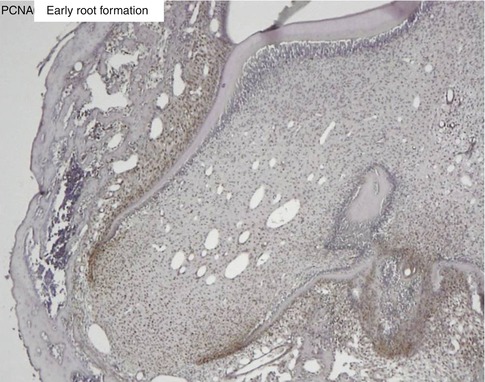

Fig. 1.10
Proliferating cell nuclear antigen (PCNA) labeling of the apical cells of the forming root. The epithelial Hertwig’s root sheath divides and elongates, contributing to root elongation and to the tooth eruption
The enamel organ, the dental papilla, and the dental follicle form the dental organ or tooth germ.
In humans, there are two dentitions, deciduous and permanent (primary, temporary). The development of the deciduous dentition begins around the sixth gestational week. Then quickly, there is coexistence of deciduous and permanent dental germs, and odontogenesis ends around the age of 18–25 years by formation of the dental root and the eruption of the third permanent molars.
The development of the permanent teeth begins during the odontogenesis of deciduous teeth. The deciduous teeth are formed from the primary dental lamina. In humans, the permanent teeth develop in two ways: (1) sequentially at the lingual region of the enamel organ of each temporary tooth (successional teeth) or (2) permanent molars grow from an extension of the initial dental lamina. These are non-successional teeth.
The spatiotemporal control of dental development is orchestrated by epithelial-mesenchymal interactions. At the molecular level, these interactions provide reciprocal and sequential exchanges of signals through the basement membrane. These signals/morphogens are associated with the “determination” (commitment) of the cells in different territories during the morphogenesis.
References
1.
2.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


