Medical oncology is an important aspect of treatment in head and neck malignancies. Specific medical therapy in head and neck malignancy is tailored to specific cell types. More than 95% of primary head and neck tumors are squamous cell carcinomas, with adenocarcinoma representing less than 5%. This chapter focuses on squamous cell carcinoma of the head and neck (SCCHN) and on salivary gland adenocarcinomas.
EPIDEMIOLOGY
According to the American Cancer Society’s Cancer Facts & Figures , approximately 34,360 new cases of cancer of the oral cavity and pharynx will be diagnosed in the United States in 2007. The incidence of head and neck cancer in men is more than twice the incidence in women. Men older than 50 years of age are at increased risk of developing head and neck cancer. The American Cancer Society estimates that 7,550 people will die of cancer of the oral cavity and pharynx in 2007.
The trend in 5-year survival rates is improving, but a disparity still exists between Caucasian and African American individuals. Five-year survival for Caucasians was 59.1% in the years 1996 to 2003 compared with 62% in 1995 to 2001; 5-year survival for African Americans was only 40% in 1995 to 2001, although this represented an improvement from 36% in 1974 to 1976. Survival rates for head and neck cancer also differ widely depending on the extent of disease, as would be expected. Five-year survival for localized disease in 1995 to 2001 was 82.1%, compared with 51.3% for regional involvement, and only 27.6% for distant metastases. This underlines the importance of early detection, particularly in the population at high risk for development of head and neck malignancy. Screening should be performed by an individual’s dentist or primary care provider during regular checkups.
Risk factors for the development of squamous cell carcinoma of the head and neck are known to include tobacco use via smoking (cigarette, pipe, and cigar) and the use of smokeless (chewing) tobacco. This risk is further compounded by excessive alcohol consumption.
Data suggest that passive smoking also increases the risk of development of squamous cell carcinoma of the head and neck. Moreover, recent investigation suggests that oral human papillomavirus (HPV) has been identified as a risk factor.
BACKGROUND
Historically, the main treatment regimen for head and neck cancer had been surgery. Radiation treatment has been used on an adjuvant basis; recently, chemotherapy also has begun to be included for palliative or adjuvant treatment, and even for definitive treatment.
Chemotherapy traditionally has been given to induce disruption of the cell cycle of cancer cells. Recent advances in our understanding of molecular biology have opened new avenues of treatment with monoclonal antibodies. Chemotherapy treatment has moved from palliation or treatment of advanced head and neck cancer to induction therapy and treatment in early disease; the benefits of this change have been shown in recent studies.
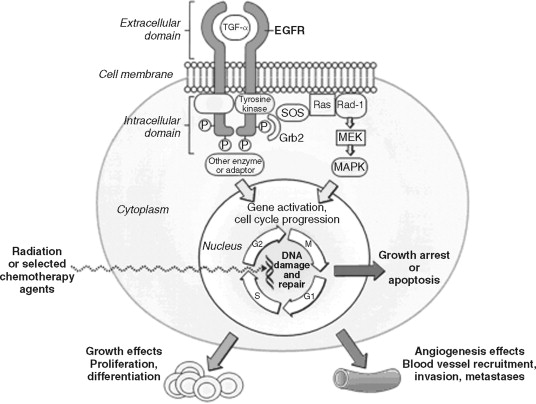
The process of carcinogenesis requires multiple genetic alterations that occur over time, producing a cumulative effect on cell division and differentiation. Proto-oncogenes are responsible for cell growth. When mutated, proto-oncogenes become oncogenes, which are perpetually activated to promote uncontrolled growth of the cell ( Figure 40-1 ).
BACKGROUND
Historically, the main treatment regimen for head and neck cancer had been surgery. Radiation treatment has been used on an adjuvant basis; recently, chemotherapy also has begun to be included for palliative or adjuvant treatment, and even for definitive treatment.
Chemotherapy traditionally has been given to induce disruption of the cell cycle of cancer cells. Recent advances in our understanding of molecular biology have opened new avenues of treatment with monoclonal antibodies. Chemotherapy treatment has moved from palliation or treatment of advanced head and neck cancer to induction therapy and treatment in early disease; the benefits of this change have been shown in recent studies.
The process of carcinogenesis requires multiple genetic alterations that occur over time, producing a cumulative effect on cell division and differentiation. Proto-oncogenes are responsible for cell growth. When mutated, proto-oncogenes become oncogenes, which are perpetually activated to promote uncontrolled growth of the cell ( Figure 40-1 ).
INDUCTION CHEMOTHERAPY IN SQUAMOUS CELL CARCINOMA OF THE HEAD AND NECK
The treatment of patients with SCCHN has become very complicated and usually requires a multidisciplinary approach. Early-stage disease can potentially be cured by surgery or radiotherapy; however, locally advanced disease or metastatic disease frequently requires the use of combined modalities, namely, surgery, radiotherapy, and chemotherapy. Over the years, it has been documented that SCCHN is one of the most sensitive malignancies to chemotherapy. Recent studies showed an overall response rate of 90% and a complete response rate of 30%. Because of this, the use of induction chemotherapy before definitive surgery and/or radiation treatment has been explored in the treatment of patients with SCCHN and has gained popularity. The object is to decrease or prevent distant metastases. Multiple phase II/III clinical trials were conducted to extensively investigate the worth of induction chemotherapy in SCCHN. These trials varied in their study design and in the chemotherapeutic agents given, as well as in their schedule of administration. However, investigators failed to show a survival benefit with induction chemotherapy.
One European trial showed an overall survival benefit for those patients who received induction chemotherapy over the control group, but event-free survival was of marginal significance. In another study, a decrease in distant metastases was evident in the group given induction systemic chemotherapy. In this study, 5-fluorouracil and cisplatin were combined; this resulted in a decrease in 3-year distant metastases from 38% to 14%. Apparent improvement in the incidence of distant metastases with the use of induction chemotherapy did not influence locoregional control. Several other meta-analytic studies failed to document survival benefit from induction chemotherapy. However, a decrease in the incidence of distant metastases after the use of induction chemotherapy for SCCHN has been documented in multiple studies. In recent years, interest in induction chemotherapy has been renewed by an improved response rate after incorporation of taxane.
The combination of mainly 5-fluorouracil, cisplatin, and taxane yielded an overall response rate greater than 90% with a complete response rate greater than 50% and a preliminary result suggestive of superiority of the three-drug combination over the two-drug combination. This could strongly suggest that use of a more effective chemotherapy combination might lead to better results in patients with SCCHN, especially with the availability of newer agents that show activity against SCCHN, such as the epidermal growth factor receptor (EGFR) blocker. Agents such as cetuximab have shown activity in platinum-refractory SCCHN as a single agent or in combination with other chemotherapy regimens. However, data on the use of EGFR blockade in the induction chemotherapy setting are not available; additional studies should be conducted to explore outcomes with this combination.
CHEMOTHERAPY OF PATIENTS WITH RECURRENT OR METASTATIC HEAD AND NECK CARCINOMA
More than 50% of patients given a new diagnosis of SCCHN will experience a recurrence after receiving primary treatment. Patients with SCCHN have a poor prognosis once primary treatment has been unsuccessful and recurrent disease has occurred. Outcomes remain very poor and have not changed over the past 30 years. Several treatment options are available for patients with recurrent disease. None of these shows survival superiority, although palliative chemotherapy showed a survival benefit over the best supportive care in one study. Multivariate analysis of data from palliative chemotherapy clinical trials has shown that time to progression and overall survival are greatly influenced by factors other than the chemotherapy agents that are used; poor performance status, prior treatments, and advanced stage of disease all are associated with a marked reduction in response to chemotherapy. Because treatment is only palliative and may be associated with significant adverse effects, clinicians must be very careful when selecting patients. The benefits must be weighed against the side effects and complications of treatment before a patient can be committed to palliative chemotherapy. Salvage surgical intervention for local recurrence has resulted in 15-30% long-term disease control. However, if the recurrent disease is inoperable, or if it is metastatic in nature, systemic chemotherapy and/or local radiotherapy should be used or considered for palliative purposes.
In the 1980s, several clinical trials compared different chemotherapeutic combinations. The outcomes of these trials are presented in Table 40-1 .
| Trial | No. of Patients | Response Rate % (CR %) | Median Survival (months) | Year of Publication | High-Grade Toxicity * |
|---|---|---|---|---|---|
| CDDP + cetuximab | 117 † | 26 V 10 | 9.2 V 8.0 | 2005 | 173 V 93 |
| CDDP + FU v CDDP + Tax | 218 † | 27 V 26 | 8.7 V 8.1; OS at 1 year, 41% v 32% | 2005 | 334 V 198 |
| MTX v docetaxel (weekly) | 57 | 15 (5) V 27 (3) | 3.7 V 3.9 | 2004 | 6 1/21 |
| CDDP + high-dose Tax ι/CDDP + conventional Tax | 210 † | 35 V 36 | 7.6 V 6.8 | 2001 | 321 V 320 |
| Raltitrexed + CDDP + FU/LV v MTX + CDDP + FU/LV | 72 | 81 (28) V 42 (8) | 2002 | 67 V 32 | |
| Edatrexate v MTX | 264 † | 21 V 16 | 6 for both groups | 1995 | 90 V 45 |
| CABO v CDDP + FU v CDP | 382 † | 34 (9.5) V 31 (1.7) V 15 (2.5) | 7.3 (TTP, 4.8 V 4.3 V 3) | 1994 | 34 V 35 V 20 |
| MTX + lonidamine | 89 | 26 (10.5) V 18 (0) | 1994 | 78 V 41 | |
| CDDP 100 mg/m 2 + FU 4 g/m 2 q21d v CBDCA 300 mg/m 2 + FU 4 g/m 2 q21d v MTX 40 mg/m 2 wkly | 277 † | 32 V 21 V 10 | 6.6 V 5.0 V 5.6 | 1992 | 66 V 47 V 35 |
| CDDP 100 mg/m 2 + FU 4 g/m 2 v CDDP 100 mg/m 2 v FU 4 g/m 2 ; all q21d | 249 † | 32 V 17 V 13 | 5.7 for all groups | 1992 | 77 V 34 V 32 |
| CBDCA + MTX v MTX | 40 † | 25 V 25 | 3 V 2 | 1989 | |
| CABO v CDDP | 209 † | 30 V 15 | 9 (TTP, 4.5) | 1988 | 57 V 11 |
| CABO v vincristine + MTX + bleomycin | 185 † | 50 (16) V 28 (5) | 9 (TTP, 4.5 V 3.5) | 1987 | 35 V 41 |
| CDDP + bleomycin + vincristine v MTX | 191 | 24 V 16 | 7.2 V 7.8 | 1986 | 43 V 57 |
| Control v bleomycin v CDDP + bleomycin v CDDP | 117 | n.a. V 14 V 24 (5) V 13 (3) | NS except CDDP arms V others 4.3 V 1.8 | 1985 | NA V 4 V 25 V 24 |
| MTX + bleomycin + CDDP v MTX | 163 † | 48 (16) V 35 (8) | 5.6 for both groups (TTP, 3.5) | 1985 | |
| CDDP v MTX | 100 † | 8 V 16 | 4.5 V 5 | 1985 | 260 V 174 |
| High-dose (1.5 g/m 2 ) MTX + LV v conventional (40 mg/m 2 ) MTX | 47 † | 32 V 22 | 4.2 for both groups | 1984 | 30 V 10 |
* Wherever toxicity was quantified by grade, relative number or percentage of grade 3 to 5 toxicities are reported. This is a sum of the worst event per patient for each toxicity type based on either absolute values or percentages reported in the publication.
† Majority of patients on this trial had prior therapy (excluding chemotherapy).
Multiagent chemotherapy in general was associated with a higher response rate. Cisplatin and multiagent regimens revealed a higher degree of toxicity. Platinum-containing combination chemotherapy yielded the highest response rates, occasionally approaching 50%. The combination of cisplatin (CDDP) plus 5-fluorouracil (5-FU) emerged as the most effective treatment for SCCHN in the 1980s and 1990s. In a three-arm study conducted by Clavel and colleagues, 5-FU and CDDP combinations were compared with carboplatin, methotrexate, bleomycin, and vincristine and with CDDP alone. Results with the CDDP plus 5-FU and carboplatin arms were superior to those with CDDP alone, with a median survival of 73 months. However, greater toxicity was encountered in all CDDP arms. When taxanes became available in the 1990s, clinical trials were undertaken to evaluate these drugs. A single taxane agent combined with paclitaxel and docetaxel produced a response rate of 30-40%. Because of the high rate of single-agent activity and the non-overlapping side effects seen with other agents, taxanes were used in clinical trials in combination with CDDP or carboplatin. These studies showed a response rate of 27-53%, with median survival of 5 to 12 months. When double-chemotherapy regimens were found to be successful, triple-chemotherapy combinations were given in an attempt to enhance response rates. Indeed, an increased response rate up to 60% and a complete response rate of 15% were attained with the combination of ifosfamide, paclitaxel, and CDDP or carboplatin. Unfortunately, side effects also were increased, which possibly defeated the purpose of palliation.
New, promising agents such as oxaliplatin have been shown to produce a 10% response rate. EGFR blockade has been investigated, but a single-agent EGFR blocker was found to produce a low response rate. Preliminary results of the chemotherapy and EGFR blocker combination do not show superiority with addition of the EGFR blocker in progression-free survival (PFS) and overall survival (OS), although a significantly higher response rate has been seen (26% vs. 10%).
In summary, the combination of CDDP plus 5-FU remains the standard of care combination for patients with SCCHN with good performance status. Although the addition of taxane may enhance the CDDP plus 5-FU combination, additional side effects may interfere with its becoming the standard of care in patients with poor performance status. Additional studies are needed to improve response rate in this patient population with a bad outcome and outlook.
ADJUVANT CHEMOTHERAPY FOR SQUAMOUS CELL CARCINOMA OF THE HEAD AND NECK
Historically, surgery has been the mainstay of treatment for SCCHN. For those patients with locally advanced disease and poor risk factors for disease, radiation treatment has been added as the main adjuvant treatment. However, low locoregional control rates have resulted, and 5-year survival rates have ranged from 10-40%. Since the 1970s, adjuvant chemotherapy has been tested both sequentially and concomitantly with radiation treatment. The rationale for inclusion of chemotherapy in the therapeutic management of locally advanced tumors is based on three primary observations:
- 1.
Even though 70-75% of patients remain free of disease at 2 years, the long-term prognosis remains poor. Five-year survival rates rarely exceed 30-35%.
- 2.
Although a fairly large number of deaths are linked to intercurrent disease, metastasis can occur at a rate from 15-20%.
- 3.
A variety of cytotoxic agents have demonstrated efficacy against epithelial cell cancers.
The use of chemotherapy alone has proved disappointing. However, compliance has not been optimal, especially after surgery and radiation treatment were provided. Evidence of a biologic effect was seen, in that the pattern of failure was associated with a decrease in distant metastases in selected studies; no convincing improvement in overall survival was apparent.
To improve the prognosis for patients with stage III to IV disease, investigators began to combine chemotherapy with radiation. In an intergroup study (0034; Lawrence GE 1992), radiation treatment was administered with or without three cycles of cisplatinum/5-FU to patients with completely resected SCCHN. This study reported no difference in OS, disease-free survival, or locoregional failure between the two groups. A significant decrease in distant metastases was seen in the chemotherapy/radiation therapy (CRT) versus the radiation therapy (RT) arm alone (15% vs. 23%, respectively; P = .03). Subgroup analysis showed that patients with high-risk pathologic features (at least two positive neck lymph nodes, extracapsular extension, and/or positive surgical margins) improved more with the addition of chemotherapy than did those in the low-risk group in terms of both tumor control and survival.
Starting in the early 1990s, analyses of clinical trials undertaken to compare CRT versus RT showed that CRT significantly increased locoregional control and survival rates.
Platinum compounds and 5-FU have been used most often in prospective clinical trials. These agents have shown anti-tumor effects but also were found to exert a radiosensitization effect (especially cisplatinum).
Various studies have tested the effects of CRT and reported associated benefits. However, considerable variability in schedule, dose, and chemotherapeutic agents used was seen in these studies. Because of this, two randomized trials conducted by the European Organization for Research and Treatment of Cancer (EORTC) and the Radiation Therapy Oncology Group (RTOG) were designed to compare the use of high-dose cisplatinum (100 mg/M 2 ) on days 1, 22, and 43, given concurrently with radiation, versus radiation alone in patients with resected, poor-risk SCCHN of the upper aerodigestive tract (high-risk patients were more broadly defined).
The EORTC study demonstrated a significant ( P = .04) difference in PFS. The primary end-point of the study favored the CRT group. Estimated median PFS was 23 months in those given RT and 55 months in the CRT group. Five-year Kaplan-Meier PFS estimates were 36% and 47%, respectively ( P = .04). OS ( P = .02) and locoregional control ( P = .07) also significantly improved. The RTOG study showed significant benefit for disease control with CRT over RT.
Disease-free survival and locoregional control rates at 2 years favored the CRT arm ( P = .04 and P = .01, respectively). Also, a trend toward higher OS rates was seen with CRT ( P = .19). No impact on distant control was noted. Both studies yielded positive results with regard to favorable impact of CRT on disease control. The magnitude of impact of chemotherapy on outcomes was greater in the EORTC than in the RTOG trial, but this may reflect variations in patient selection.
In a recent publication, data from EORTC and RTOG were analyzed. Extracapsular spread (ECS) and/or microscopically involved surgical margins revealed the most significant impact of CRT. A favorable trend toward CRT was seen in patients with stage III to IV disease, perineural infiltrates, vascular embolism, and/or clinically enlarged lymph nodes at level IV or V associated with tumors in the oral cavity or the oral pharynx. Patients with two or more histologically involved lymph nodes who did not exhibit ECS or positive margins as additional risk factors did not appear to benefit from the addition of chemotherapy in this analysis.
CHOICE OF CHEMOTHERAPY IN THE TREATMENT OF PATIENTS WITH HEAD AND NECK CANCER
Over the past three decades, interest in the medical management of SCCHN has increased. This trend has stemmed from the realization that this group of malignant tumors is unusually sensitive to chemotherapy. Numerous single agents have been found to be effective against SCCHN ( Table 40-2 ).
| Agent | No. of Patients Assessable | Response Rate | Median Survival (months) RR (%) | Year of Publication |
|---|---|---|---|---|
| Methotrexate | 8-77 (average 31) | 1984 | ||
| Bleomycin | 6-45 (average 21) | 1977-1984 | ||
| Cisplatin | 14-41 (average 28) | 1983-94 | ||
| Carboplatin | 25 | 1986 | ||
| Oxaliplatin | 10 | 1996 | ||
| Cyclophosphamide | 36 | 1980 | ||
| Doxorubicin | 24 | 1980 | ||
| Hydroxyurea | 18 | 39 | 1980 | |
| Vinblastine | 29 | 1980 | ||
| Vinorelbine | 6 | 1994 | ||
| Fluorouracil | 15 | 1984 | ||
| Gemcitabine | 61 | 13 | 1994 | |
| Capecitabine | 14 | 8 | 2003 | |
| Orzel | 42 | 21 | 2001 | |
| Irinotecan | 0-14 | 2005 | ||
| Paclitaxel 24-hour infusion | 34 | 40 (4 CRs) | 9.2 | 1998 |
| Paclitaxel 96-hour infusion | Chemotherapy naïve/paclitaxel naïve/paclitaxel exposed | 13/0/0 | 5.5 | 2004 |
| Docetaxel | 21-42 | 1994-2005 | ||
| Permetrexed | 35 | 26 | 6.4 | 2001 |
| Ifosfamide | 26 | 2003 | ||
| Cetuximab | 103 | 13 | 2005 | |
| Erlotinib | 115 | 4 | 2004 | |
| Gefitinib | 47 | 11 | 8.1 | 2003 |
| Sorafenib (BAY 43-9006) | 10 | 6 SD (60%); 4 SCCHN + 2NPC; range, 3-6 cycles | 2005 |
To enhance patient response to chemotherapy, multiple randomized clinical trials were designed to test the efficacy of combination chemotherapy in previously treated patients with locoregional failure. Results were favorable for combination chemotherapy, with a consistent 30-40% response rate (see Table 40-1 ).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


