Introduction
The aim of this study was to investigate clinically and histologically the efficiency of self-drilling microimplants as orthodontic anchorage with immediate, continuous, and constant loadings.
Methods
Titanium-alloy microimplants with diameters of 1.2 to 1.3 mm were manually placed into the buccal sides of both jaws, including the interradicular areas, in 3 dogs. Implants were placed without predrilling in thin cortical bone areas; in thick cortical bone areas, a 2-mm deep pilot hole was drilled. Thirty-six microimplants, subjected to approximately 200 g of immediate horizontal loading, served as the study group. The remaining 8 received no loading and were the controls over the 9-week observation period. The distances of reciprocally loaded microimplants and crevicular pockets were measured at the beginning and end of loading. Serially undecalcified and decalcified sections of the microimplants and surrounding tissues were studied with light and fluorescent microscopes. After 9 weeks of observation, 22 fixtures were easily removed with a screwdriver. Two were broken, and 1 was movable.
Results
Histologic analysis showed good osseointegration in all stable samples, and new bone formation and bone apposition to the surface of the threads in loaded and unloaded samples. Histomorphometric evaluation showed high bone-to-implant contact values in the loaded samples, but no significant statistical differences from the unloaded ones.
Conclusions
Titanium alloy microimplants with small diameters (1.2-1.3 mm) are strong enough for self-drilling and immediate loading in thin cortical bone areas, but, to reduce the chance of breakage, a drilling of a pilot hole is suggested in thick cortical bone areas.
It is not an exaggeration to say that temporary skeletal anchorage is a revolutionary orthodontic technique, because anchorage control is a functional concept in clinical treatment. As a successful routine anchorage device, it simplifies orthodontic treatment, eases the patient’s pain after surgery, and is an efficient treatment method. However, when immediate or early loading is needed, its stability is challenged because of conventional placement methods. The stability of self-tapping miniscrew implants has been reported to be clinically acceptable but not absolute before ossointegration is achieved.
One reason for displacement might be early bone resorption after surgical trauma and early loading. Some intermediary procedures have been proposed after implant bed preparation to enhance the progress of bone healing. Not only is nontraumatic surgery essentially considered, but also several conditions should be included, such as changing the drilling method to reduce original bone damage, chemical modification to increase bone modeling and remodeling, and adding implants to decrease micromovement.
With the improvements in biomaterials, various implants have been designed to be smaller and easier to handle, with less use of instruments. A recently developed form of implants, self-drilling microscrew implants (SDIs), which can be placed without predrilling and no incision, have the advantages of predrilled implants without the potential disadvantages of burning tissues. Less bone damage helps to prevent bone necrosis and screw loosening, and also improves the success rate. Saving time is another advantage of drill-free microimplants.
However, because of limited data about SDIs, their clinical characteristics are unclear, and histologic changes, in particular, with immediate loading are unknown. The issues of what SDIs are as acceptable as predrilled fixtures have been raised, since self-drilling was recommended in some studies. It was stressed that SDIs have a stronger resistance to placement than self-tapping microimplants (STIs). It has been reported that greater bone damage occurs during the placement of self-drilling screws compared with STIs.
The purposes of this study were to examine the potential of SDIs under immediate orthodontic loading clinically and to evaluate the possible damage to bone histologically.
Material and methods
The experimental protocol was approved by the Animal Care Committee of the Medical College at Kyungpook National University, Daegu, Korea. The experimental subjects, assessment methods, and criteria were the same as in our previous STI study. Briefly, 3 female mongrel dogs, 1 year old, with similar weights (14 ± 1 kg), were used to minimize the difference of physical bone reaction. All procedures were performed under sterile conditions, and the animals received general anesthesia with intramuscular injections of a ketamine cocktail. Each dog’s mouth was cleaned with 0.2% chlorhexidine gluconate solution fortnightly.
Forty-four self-drilling, machined-threaded titanium alloy (Ti-6Al-4 V) microimplants (Absoanchor, Dentos, Daegu, Korea; SH1312-07, 1.2-mm tip diameter, 1.3-mm neck diameter, 7-mm length) were manually placed with a hand driver made especially for them without incisions. During placement, 3 implants at the inferior and posterior area of the mandible were broken (2 in dog A, 1 in dog C), so, in a thick cortical bone area, an approximately 2-mm deep pilot hole was drilled. Eighteen pairs of implants were unsubmerged in both jaws and immediately loaded with approximately 200 g of continuous and constant force by stretching 8-mm superelastic closed nickel-titanium springs (Tomy, Tokyo, Japan) for 9 weeks; they were the test group. One microimplant at the maxillary left second premolar area of dog A loosened during spring placing. The remaining 8 unloaded fixtures (2 in dog A, 4 in dog B, 2 in dog C) were placed at the buccal sides of both jaws as controls ( Fig 1 ). Another 4 microimplants were replaced in addition to the broken and loosened ones.
Mobility of the microimplants was measured with dental tweezers and recorded on a 3-grade scale in which 0 denoted no mobility and was defined as a success. Grade 1 indicated palpable mobility, and grade 2 meant visible mobility; both were defined as failures. The mobility examinations were performed before and after the observations.
Crevicular pocket depth was measured on the mesial and distal aspects of the test and control implants with a custom-made periodontic probe before and after loading.
For the assessment of implant dislocation, the distances between the reciprocal loaded pair implants were clinically and directly measured with a sharp-point digital sliding micrometer (Ortho114, Seoul, Korea) in each dog’s mouth before and after loading. Every measurement was made twice, and the mean value was taken. The dislocation was decided by dividing the difference of the 2 readings before and after loading.
Fluorochrome bone labeling was used to assess the rates of bone modeling and remodeling. Each dog was given doses of oxytetracycline (25 mg per kilogram per day of body weight; Kepro Oxytet, Barmeveld, the Netherlands) for the first 4 days after the implants were placed. One dose of calcein (25 mg per kilogram per day of body weight; SIGMA, Steinheim, Germany) was subcutaneously administered at the fourth week. A dose of 0.16% alizarin red (75 mg per kilogram per day of body weight; SIGMA) was intramuscularly injected at the seventh week.
All retrieved specimens that would be subject to decalcified and undecalcified evaluation were fixed in 10% formaldehyde for 48 hours at room temperature and then changed to phosphate-buffered saline solution (pH 7.4). For assessing bone apposition, bone remodeling, and bone osseointegration, 15 specimens were stained with Villanueva solution and embedded in polyester resin after they were dehydrated and defatted in graded ethanol. Longitudinal sections of the bone-implant interface were cut at thicknesses of 500 μm with a low-speed digital saw (Struers Accutom-50, Bellerup, Denmark) and ground to 50 μm until the implants and surrounding tissues could be clearly seen with light and fluorescent microscopes.
Four specimens were stained with hematoxylin and eosin for assessing the orientation of the surrounding bone and root damage.
Histomorphometric analysis was performed on combined images including the entire implant surface. The micrograph was spliced by using MagicScan32 (version 4.6, SPSS Science, Chicago Ill). Each implant consisted of 7 magnification (100 times) objectives (Olympus BX51, Tokyo, Japan) from the Villanueva-stained specimens. The spliced implant images were quantitatively analyzed by using computer-assisted image-analysis software (Image & Microscope Technique, Calif) to calculate the percentage of bone-to-implant contact (BIC) on the mesial and distal aspects of the implants. Two ways were used for expressing the percentages of BIC contact. The first way was used for considering the ratio of the length of the bone contact area to the total length of the implant interface. The second was for calculating the ratio of the length of bone contact area to the length of the implant interface in the cortical bone area.
Results
The dogs remained in good health without obvious weight loss during the experimental period and with no complications in the whole procedure. During placement, 3 implants fractured at the caudal aspect of the head, and 1 loosened during loading. Broken and loose implants were substituted for the supplemented 4 microimplants for the analysis of success rate. Other miniscrew implants of the 2 groups had primary stability, and most remained stable for the 9-week observation period, except for a loose one (in dog A) in grade 2 and 2 broken implants (1 in dog A, 1 in dog C) at the fourth week. The success rate was 91.67% in the loaded group, with no failures in the unloaded group. The distribution of the microimplants is shown in Figure 2 .
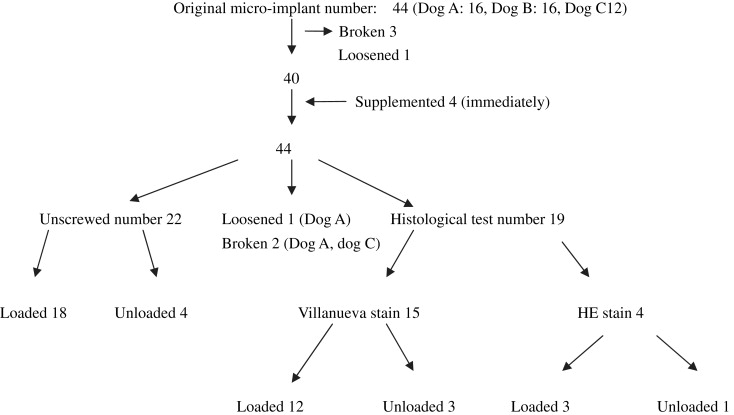
At the end of the observation period, 18 miniscrew implants in the loaded and 4 unloaded samples were unscrewed with a screwdriver easily, and no breakage occurred, even without soft and hard tissues being abstracted.
No pockets of the peri-implant were seen at the mesial and distal sides of the implants of the 2 groups. The gingiva around the unloaded implants was observed to be clinically healthier than that around the loaded implants.
The mean displacements of the loaded implants were 0.36 mm in the maxilla and 0.18 mm in the mandible, ranging from –0.03 to 0.58 mm. For the unloaded samples, only 4 implants at the posterior buccal side of dog B were measured, and a –0.13-mm displacement was found.
After the soft tissues were removed, 3 to 5 threads of the microimplants at the anterior buccal sides of both jaws were found out of the bones. The findings from the light microscope for the slides stained with hematoxylin and eosin showed a little irregular bone direction at the interface of surrounding bone, but no root damage was visible.
Through a general light microscope for the Villanueva-stained specimens, most of them were monocortical. On average, 5.5 threads of the microimplant were in cortical bone, ranging from 2 to 8 threads, because of the extrusion and the bone marrow cavity.
Osseointegration between implants and surrounding bone was seen in all stable samples. Apposition was slightly more in the loaded samples. Proliferation was seen at the neck and the marrow side in the unloaded samples, which were composed of thin, compact bone and had a highly porous, spongy inner structure ( Fig 3 ).
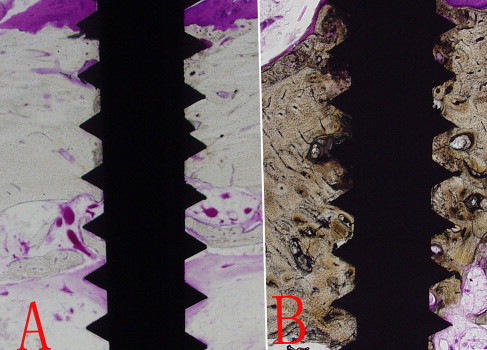
Under the high magnification of a general light microscope, the findings from the longitudinal sections of the Villanueva-stained specimens showed less complete osseointegration in the unloaded specimens ( Fig 4 ). The microimplants were primarily osseointegrated with cortical alveolar bone, and no fibrous tissue intervention was observed in the 2 groups. More mature trabecular bone and new bone interspersed with less bone resorption were generally observed at the bottom of the threads in the loaded specimens.




