Fig. 14.1
Number of publications (English language) on the PDT since 1980 till 2010 (Source—Pubmed)
Mechanism of Photodynamic Inactivation of Microbial Cells
Antimicrobial photodynamic therapy works as a combination of photosensitizer and light. Photosensitizer is a light-sensitive chemical that possesses low toxicity in the absence of light. Photosensitization of the infected tissue with a photosensitizer allows uptake into the bacterial cells, and irradiation of the photosensitized tissue results in the destruction of bacteria and infected tissue. It is extremely important that the light should be at a specific wavelength, which corresponds to the absorption wavelength of the photosensitizer being used. PDT can be utilized with a suitable photosensitizer and irradiation conditions to treat infections in cases where antibiotic-based therapeutic strategies have failed [8]. Unlike in cancer therapy where the photosensitizer is administered intravenously, for localized infections, the photosensitizer is delivered locally by various methods such as topical application, instillation, and interstitial injection or aerosol delivery. Selectivity of photosensitizer toward microorganisms over mammalian cells and effective removal of the causative microorganisms are the key points in achieving success of PDT to manage localized infections [7].
Photosensitizers are chemicals, when excited, capable of transferring the energy absorbed to other compounds in the vicinity that, in turn, generate very reactive metastable species. The triplet-excited state of the photosensitizer releases energy to come to the ground state via two specific mechanisms: type I or type II pathway [18]. Type I pathway involves production of radical ions of oxygen due to electron transfer from the photosensitizer triplet-excited state to the substrate. Radical ions such as superoxide, hydroxyl, and lipid-derived ions are the cytotoxic species responsible for type I photoreaction [19]. Type II pathway involves production of excited singlet oxygen due to energy transfer from the photosensitizer triplet-excited state to the ground-state molecular oxygen, which is responsible for the oxidation of various cellular constituents [20]. The antimicrobial effect of PDT is mainly due to type II reaction. Singlet oxygen is a strong oxidizing agent and thus highly reactive, with a lifetime of less than 0.04 μs in a biological environment and a radius of action of less than 0.02 μm [21]. The reactions of singlet oxygen with the cellular targets lead to cell death. The above two basic mechanisms account for this lethal damage to the bacterial cell by DNA damage and cytoplasmic membrane damage. Figure 14.2 shows the photodynamic inactivation of bacterial cells in a stepwise manner. It should be noted that the differences in microbial cell wall characteristics and bacterial growth mode should be accounted while determining the duration of photosensitization before light illumination [22, 23]. The photosensitizer with slower uptake could result only in cell wall damage and with longer incubation times; other nuclear effects such as nucleic acid strand breakage might be apparent. The choice of photosensitizer is thus critical in obtaining effective bacterial elimination.
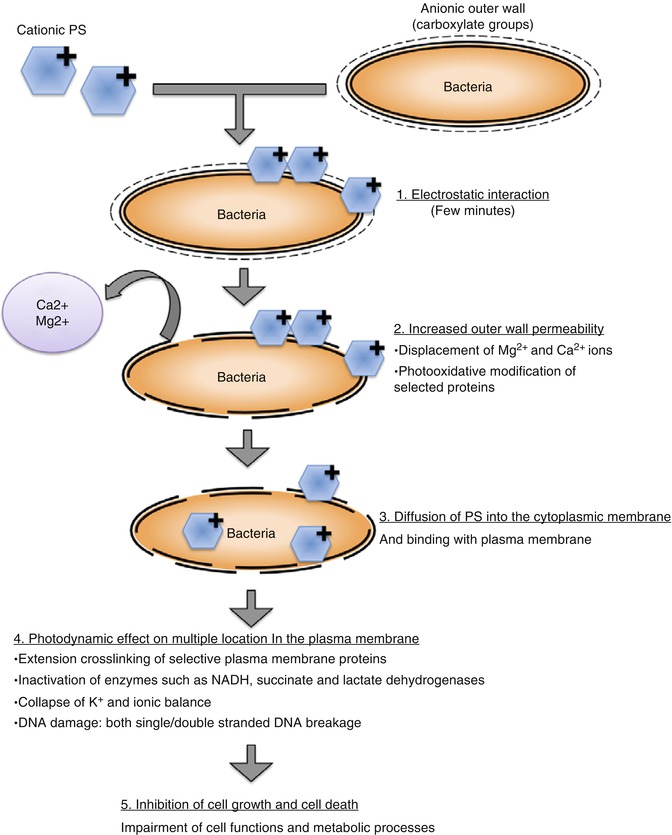

Fig. 14.2
Schematic showing the stepwise mechanism of photodynamic inactivation of microbial cells
One of the significant advantages of PDT is the targeted antibacterial effect. Choosing a photosensitizer that has high affinity for microbial cells and irradiating the specific area of infection could result in the targeted effect of antimicrobial PDT. As the photosensitizer typically shows a higher affinity toward microbial cells, the host cells could be affected less during PDT. Toxicity of the photosensitizer usually occurs when high concentration/volume of photosensitizer is applied to a tissue to obtain more significant treatment response. The instant antimicrobial activity also offers added advantage as antibiotics take several days to produce comparable efficacy. The broad therapeutic window of PDT because of the high reactivity of ROS could effectively eliminate bacteria as well as the bacterial virulence factors such as endotoxins and proteolytic enzymes. Furthermore, due to the multiple targets of PDT on a bacterial cell, the probability of bacteria developing resistance to this treatment has been considered to be almost impossible [7, 8, 24].
Photosensitizers such as porphyrins, chlorins, and phthalocyanines, for treatment of cancer or other diseases, are chosen based upon their low dark toxicity to mammalian cells and ability to target tumor cells [8]. The photosensitizers for antibacterial PDT are chosen based on their specificity to bacterial cells. Large numbers of photosensitizer potentially useful in LAD are currently in various stages of clinical trials for FDA approval. The commonly used photosensitizers for antibacterial purpose are halogenated xanthenes such as rose bengal (RB) [25], phenothiazines such as methylene blue (MB) and toluidine blue (TBO) [9, 22, 26], and perylenequinones such as hypericin [27]. The factors that determine the effectiveness of antibacterial PDT are method/vehicle of topical application, effective time of interaction with the microbes at the site of infection, selectivity of the photosensitizer to microbes, relative non-toxicity toward host tissues at the site of infection, and ability to eliminate the microbes effectively to avoid regrowth of surviving pathogens following treatment [8].
Antimicrobial PDT on gram-positive and gram-negative bacteria induced breaks in both single and double-stranded DNA and the disappearance of the plasmid supercoiled fraction [28, 29]. In addition, the photooxidative effect caused by the phenothiazinium photosensitizer in bacteria led to the damage of multiple targets in bacterial cells such as DNA [28], membrane integrity [30], protease activity, and lipopolysaccharide (LPS) [31]. George and Kishen reported functional impairment of cell wall, extensive damage to chromosomal DNA, and degradation of membrane proteins following methylene blue-mediated APDT of E. faecalis [32]. These findings support the hypothesis that antimicrobial PDT is a feasible alternative to antibiotics since the mode of action is markedly different from that typical of most antibiotics and chances of resistance are potentially none.
Antimicrobial PDT in Root Canal Disinfection
The use of PDT in conjunction with conventional root canal disinfection methods resulted in significantly better bacterial elimination as compared to either of these treatments when used alone. Over the years, various efforts were made to optimize different PDT-related parameters for endodontic application. Several in vitro and in vivo studies have shown the effectiveness of PDT in eliminating root canal biofilms [9, 33–42]. Endodontic pathogens such as E. faecalis, P. intermedia, F. nucleatum, S. intermedius, and A. actinomycetemcomitans have been shown to be killed by using photosensitizers such as methylene blue (MB), toluidine blue (TBO), and rose bengal (RB) [43–46].
Currently PDT is not considered a replacement for the existing root canal disinfection protocols but rather considered as a potential adjunct to improve antibiofilm efficacy following current disinfection protocols during the root canal treatment. Meire et al. [47] and George and Kishen [41, 43] used antimicrobial PDT to enhance the root canal disinfection. They showed that antimicrobial PDT could effectively kill biofilms of E. faecalis with photosensitizers such as MB and TBO along with red light. Soukos et al. conducted PDT experiments on a range of endodontic pathogens (methylene blue as photosensitizer) and reported complete elimination of all bacteria except E. faecalis (53 %) [34]. In yet another study, significant antibacterial effects on suspensions of S. intermedius, P. micros, P. intermedia, and F. nucleatum were reported by Williams et al. following PDT with TBO and red light [44]. Different in vivo studies that examined the efficacy of antimicrobial PDT in root canal disinfection have been summarized in Table 14.1 [26, 36–38]. These studies concluded that a combination of chemomechanical preparation and PDT would bring about maximum reduction in microbial loads.
Table 14.1
Table showing clinical studies where PDT was used for root canal disinfection
|
No
|
Author/date
|
Objective and materials
|
Methodology
|
Conclusion
|
|---|---|---|---|---|
|
1
|
Bonsor et al. (2006) [36]
|
Aimed to evaluate the antimicrobial efficacy of root canal disinfection by combining conventional endodontic treatment with PDT
Clinical study on 32 root canals from 14 patients
|
Irrigation with 20 % citric acid and 2.25 % sodium hypochlorite
PDT with TBO and diode laser (12.7 mg/L−1, 100 mW, 120 s)
Samples collected by filing
|
Cleaning and shaping resulted in complete bacterial killing in 86.7 % of samples
Combination of cleaning and shaping + PDT resulted in complete bacterial killing in 96.7 % of samples
|
|
2
|
Bonsor et al. (2006) [26]
|
Aimed to compare the effect of a combination of 20 % citric acid and PDT with the use of 20 % citric acid and 2.25 % sodium hypochlorite on bacterial load in prepared root canals
64 patients were used
|
Procedure similar to previous study
|
Combination of 20 % citric acid and PDT resulted in complete bacterial killing in 91 % of samples
20 % citric acid and 2.25 % sodium hypochlorite resulted in complete bacterial killing in 82 % of samples
|
|
3
|
Garcez et al. (2008) [38]
|
This study analyzed the antimicrobial effect of PDT in association with endodontic treatment
20 patients were selected
First session of cleaning and shaping + PDT
At the end of first session, the root canal was filled with Ca(OH)(2), and after 1 week, a second session of PDT was performed
|
Irrigation with 2.5 % sodium hypochlorite, 3 % hydrogen peroxide, and 17 % EDTA
PDT with polyethylenimine (PEI) chlorin (e6) conjugate (2 min, 9.6 J, 240 s)
Paper point sampling
|
First session produced 98.5 % bacterial reduction (1.83 log reduction)
Second session produced 99.9 % bacterial reduction (1.14 log reduction)
Second session PDT was observed to be more effective than first session
|
|
4
|
Garcez et al. (2010) [37]
|
Studied antimicrobial effect of PDT combined with endodontic treatment in patients with necrotic pulp infected with microflora resistant to a previous antibiotic therapy
30 teeth from 21 patients with periapical lesions that had been treated with conventional endodontic treatment and antibiotic therapy were selected
|
PDT used polyethylenimine chlorin (e6) as a photosensitizer and a diode laser (40 mW, 4 min, 9.6 J)
|
Endodontic therapy alone produced a significant reduction in numbers of microbial species (only 3 teeth were free of bacteria)
The combination of endodontic therapy with PDT eliminated all drug-resistant species and all teeth were bacteria-free
|
Singlet oxygen is known to diffuse approximately 50 nm [18]. This emphasizes the close proximity of a photosensitizer molecule to the bacterial cell surface that allows diffusion of singlet oxygen. In a biofilm, only 30 % of the total mass is bacteria and remaining is the self-secreted extracellular polymeric matrix. The ability of the photosensitizer to diffuse and uniformly distribute in the biofilm structure is important for effective killing efficacy [48]. This clearly could be seen in the higher level of energy required to eliminate bacterial biofilms as compared to the planktonic counterparts [22, 46, 48, 49]. Bacteria existing in biofilms are also known to express active efflux pumps that confer their ability to transport amphiphilic chemicals and photosensitizers outside the cell [50]. This is the protective mechanism exerted by the cell to expel potentially toxic compounds. Both prokaryotic and eukaryotic cells possess various membrane proteins termed efflux pumps. Use of efflux pump inhibitors (EPI) such as verapamil would restore the antibacterial activity of a compound that is specific to an efflux mechanism. Both the phenothiazinium dyes such as MB and TBO are amphipathic cations that are potential substrate for multidrug efflux pumps [51]. Use of EPI with phenothiaziniums resulted in significantly enhanced biofilm elimination at much lower PDT dosage [45, 52, 53]. Since efflux pumps are highly active in bacterial biofilms, use of EPI could potentially enhance the antibiofilm efficacy of PDT inside root canals. Kishen et al. have demonstrated the enhanced ability of EPI in combination with MB photosensitizer to disinfect biofilm bacteria as well biofilm-derived bacteria [45, 52].
In addition to the limitations associated with the interaction/uptake of photosensitizer by intracanal bacterial biofilms, tissue-specific constraining factors in the application of PDT for endodontic disinfection also need special consideration. Some of the tissue-specific constraining factors in the application of PDT for endodontic disinfection are the limited penetration of the light energy into the infected tissue, lack of optimum photosensitizer concentration within the infected tissue, low oxygen tension inside the root canals, and dentin discoloration by the photosensitizer. These issues need to be addressed before establishing PDT as a definitive treatment step in root canal disinfection [33, 41].
In biological tissue, absorption of light is mainly due to the presence of free water molecules, proteins, pigments, and other macromolecules. The absorption coefficient strongly depends on the wavelength of the incoming light/laser irradiation. Scattering of light in tissue has the utmost effect on light intensity and directionality. Scattering, together with refraction, causes a widening of light beam, resulting in the loss of fluence rate (power per unit area) and a change in directionality of the light beam. Tissue-specific approach has been highlighted by George and Kishen, which improved the antimicrobial efficacy of PDT in root canal system. Methylene blue was dissolved in different formulations such as water, 70 % glycerol, 70 % poly ethylene glycol, and a mixture of glycerol-ethanol-water (MIX) in a ratio of 30:20:50 and analyzed for the photophysical, photochemical, and photobiological characteristics [43]. The aggregation of methylene blue molecules was significantly higher in water when compared to other formulations. In addition, the MIX-based methylene blue formulation had effective penetration into dentinal tubules and enhanced singlet oxygen generation, which in turn improved bactericidal action. A significantly higher impairment of bacterial cell wall and extensive damage to chromosomal DNA were observed when methylene blue in a MIX-based formulation was used and when compared to water [32]. The same group also showed that the incorporation of an oxidizer and oxygen carrier with photosensitizer formulation in the form of an emulsion would produce significant photooxidation capabilities, which in turn facilitated comprehensive disruption of matured endodontic biofilm structure [41].
Antimicrobial PDT has the potential to destroy microbial cells as well as mammalian cells. However, the selective killing of microbial cells over host cells is specific to the photosensitization periods and light fluence required for the antimicrobial effects. Soukos et al. compared the effect of PDT using a combination of toluidine blue O (TBO) and red light against S. sanguis and human gingival keratinocytes and fibroblasts. They reported no reduction in the human cell viability, whereas the bacteria were effectively killed [54]. Soncin et al. reported the selective killing of S. aureus over human fibroblasts and keratinocytes (four to six fold) when subjected to PDT using cationic phthalocyanine and relatively low light fluencies [55]. George and Kishen demonstrated a 97.7 % killing of Enterococcus faecalis compared to a 30 % human fibroblast dysfunction following methylene blue-mediated PDT [9]. Even the newer photosensitizer-conjugated chitosan nanoparticles showed favorable cell survival (fibroblasts) as compared to highly effective antibiofilm properties [48, 56]. All these in vitro studies suggested the targeted killing efficacy of antimicrobial PDT.
Conjugating photosensitizer to various agents or chemical moieties can result in improved photosensitizers for PDT. These modified photosensitizers are expected to bind more effectively to the outer membrane of bacteria and upon activation of generated reactive oxygen species, which then diffused into the cells, resulting in cell death. Therefore, photo-generated oxidative species are well confined to the cell wall and its vicinity, which is a highly susceptible domain for photodynamic action. Soukos and coworkers formed a hypothesis that by covalently conjugating a suitable photosensitizer to a poly–l–lysine chain, a bacteria-targeted photosensitizer delivery vehicle could be constructed that would efficiently inactivate both gram-positive and gram-negative species [57]. This was demonstrated by preparing a conjugate of chlorin (e6) and a poly–l–lysine chain (20 lysine residues), which after 1 min incubation and illumination with red light killed >99 % of the gram-positive Actinomyces viscosus and gram-negative Porphyromonas gingivalis [58]. Conjugates of polyethylenimine and chlorin (e6) when used as a photosensitizer eliminated all the drug-resistant bacteria during retreatment in failed root canal-treated teeth [37]. This PEI-ce6 conjugate eliminated both gram-positive and gram-negative bacteria in vitro and in vivo as compared to the commonly used photosensitizer TBO [59]. Anionic photosensitizer (rose bengal) conjugated with positively charged chitosan has also been shown to be highly effective in removing biofilms of gram-positive, gram-negative, and multispecies bacteria [48, 60, 61] (Fig. 14.3). Shrestha et al. showed that the rose bengal-conjugated chitosan presented a synergistic effect of the antimicrobial polymer chitosan and singlet oxygen that was generated following photoactivation [48, 56]. The chitosan-conjugated rose bengal nanoparticles (CSRBnp) penetrated deep into the biofilm structure and photoactivation resulted in total elimination of the multispecies biofilms of bacteria associated with endodontic infection [61]. These modified photosensitizers in nano-form were found to envelope the bacterial cells within minutes of the photosensitization period (Fig. 14.4) [48]. Irradiation of these bacteria with closely adhered CSRBnp resulted in total killing with various stages of membrane damage as well as release of cell constituents.
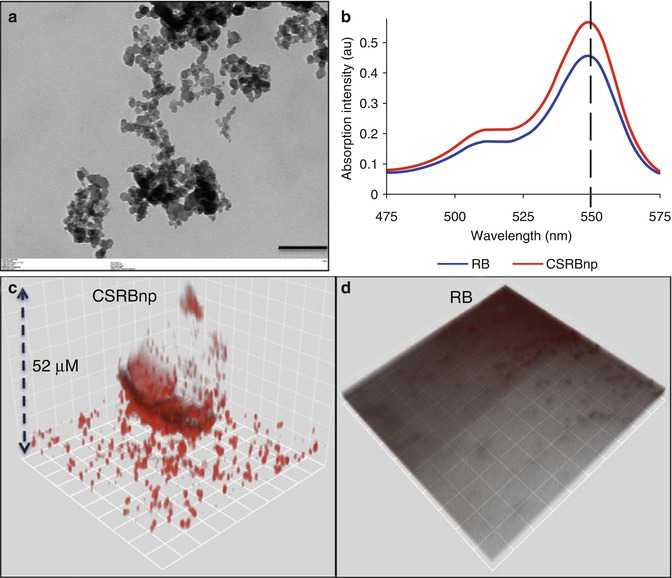
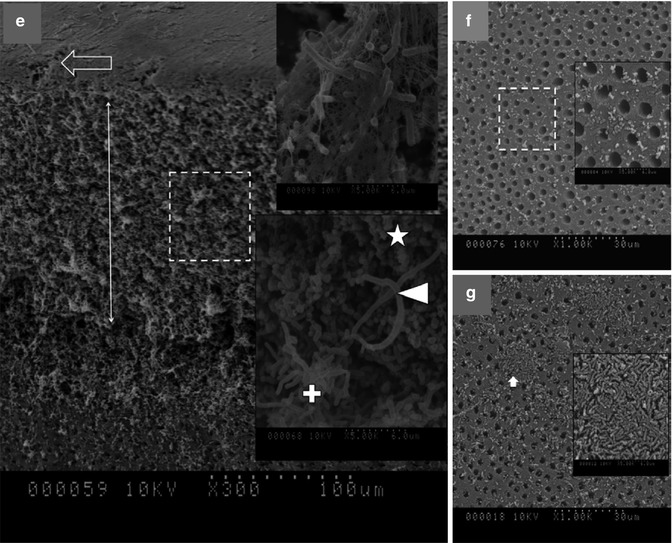
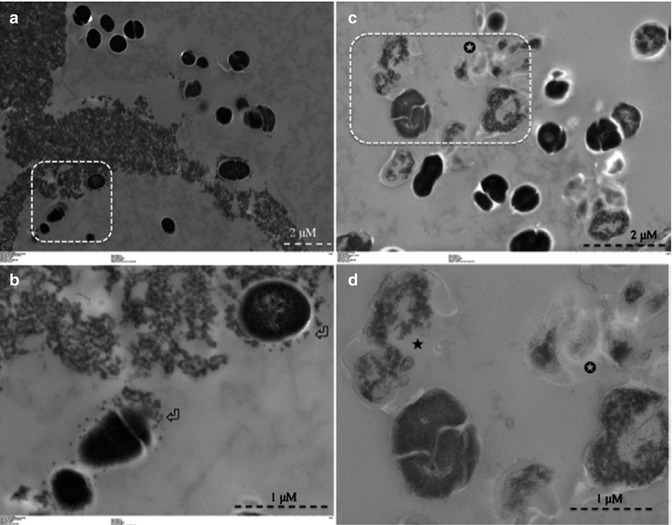


Fig. 14.3
(a) Transmission electron microscopic image of CSRBnp (scale bar = 200 nm). The CSRBnps were 60 ± 20 nm in size. (b) A typical graph showing the absorption spectrum of RB and CSRBnps. The absorption peak at 550 nm was not affected after conjugation of CSRBnps with RB. (c, d) The uptake of CSRBnps and RB into the E . faecalis biofilms as observed under CLSM. (e–g) Scanning electron microscopic images of multispecies biofilms on dentin sections. (e) The 3-week-old biofilms presented as a uniformly thick matlike structure covering the entire dentin surface. Three specific bacterial morphologies are evident in higher magnification (Denoted by *, + and block white arrowhead). The surface showed an abundant polymeric matrix (open arrowhead) (magnified area shown by the open arrow). (f) CSRBnp treatment rendered the dentin surface clean of the biofilm with open dentinal tubules. (g) RB treatment showed cleaner areas of dentin along with dense bacterial aggregates (inset: magnified area shown by the white arrow) (Adapted with permission from Shrestha and Kishen [61])

Fig. 14.4
Transmission electron microscopy images for planktonic E. faecalis after treatment with CSRBnp for 15 min (a, b). Aggregates of CSRBnp could be seen surrounding the bacterial cell. Nanoparticles were found attached to the bacterial cell surface and forming an envelope (open arrows) (b). The cells did not show any disruption of morphology. Following PDT of the sensitized bacteria, various stages of membrane damage as well as release of cell constituents were evident (c, d). Most of the bacteria showed some kind of cell membrane disruption (black star) and release of cell constituents at higher magnification (d) (Adapted with permission from Shrestha et al. [48])
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


