Introduction
The aim of the study was to quantitatively and qualitatively analyze changes in the gingival crevicular fluid (GCF) during orthodontic movement by assessing the levels of cross-linked N-telopeptides of type I collagen marker (NTx) and osteocalcin (OC) activity.
Methods
Twenty patients requiring all first premolars to be extracted were selected and treated with conventional straight-wire mechanotherapy. The canines were retracted with closed-coil springs. The maxillary canine on one side acted as the experimental site, and the contralateral canine was the control. GCF was collected from around the canines before retraction, and 1 hour, 1 day, 7 days, 14 days, and 21 days after retraction. GCF NTx and OC levels were estimated and compared with the control side.
Results
The results showed statistically significant changes in NTx and OC levels on days 7, 14, and 21 when we compared the experimental and control sides. The peak in all activity of the variables occurred on day 14 after retraction.
Conclusions
The study showed that NTx and OC levels can be successfully estimated in the GCF, and its increased levels might indicate the active tooth movement phase in orthodontic therapy.
Tooth movement by orthodontic treatment is characterized by remodeling changes in dental and periodontal tissues, including dental pulp, periodontal ligament, alveolar bone, and gingiva. These force-induced strains alter the periodontal ligament’s vascularity and blood flow, resulting in local synthesis and release of various key molecules, such as neurotransmitters, cytokines, growth factors, colony-stimulating factors, and arachidonic acid metabolites. These molecules can evoke many cellular responses by various cell types in and around teeth, providing a favorable microenvironment for tissue deposition or resorption. The biomechanical principles of tooth movement during orthodontic treatment have been extensively described. These are supported by several studies that evaluated periodontal changes incident to orthodontic tooth movement.
The early phase of orthodontic tooth movement involves an acute inflammatory response, characterized by periodontal vasodilatation and migration of leukocytes out of the capillaries. A reflection of these phenomena can be found in the gingival crevicular fluid (GCF) of moving teeth, with significant elevations in the concentrations of inflammatory mediators, such as cytokines and prostaglandins. Cytokines can provoke the synthesis and secretion of numerous substances that form the molecular basis for cell-to-cell communication, including prostaglandins and growth factors, thus interacting directly or indirectly with bone cells. The pleiotropic actions of cytokines include various effects on cells of the immune system and modulation of inflammatory responses. Because of the number and complexity of these factors, and their overlapping activities and multiple biologic effects, the assessment and interpretation of cytokine levels should be done with caution.
GCF arises at the gingival margin and can variously be described as a transudate or an exudate. Several studies have focused on the composition of GCF and the changes that occur during orthodontic tooth movement. GCF component analysis is a noninvasive method for studying the cellular response of the underlying periodontal ligament during orthodontic treatment.
A wide variety of substances involved in bone remodeling and produced by the periodontal ligament cells in sufficient quantities to diffuse into the GCF have already been studied. The mechanism of bone remodeling during orthodontic treatment is related on the one hand to the release of inflammatory mediators, such as prostaglandin-E2 and interleukin-1 β, and on the other hand to the production of neuropeptides, such as substance P and interleukin-1 β, a known potent cytokine produced mainly by activated monocytes, that participates in the initiation of bone resorption, by either activating osteoclasts or stimulating the synthesis of prostaglandin-E2.
It is well known that, when a force is applied to a tooth, the periodontal tissues undergo either tension or compression stress, depending on the tooth movement.
The discovery of cross-linked N-telopeptides of type I collagen (NTx) has provided a specific biochemical marker of human bone resorption that can be analyzed by immunoassay. The NTx molecule is specific to bone due to the unique amino acid sequences and orientation of the cross-linked alpha-2 N-telopeptide. Generation of the NTx molecule is mediated by osteoclasts or bone and is found in urine and serum as a stable end-product of degradation.
Osteomark NTx serum provides a quantitative measure of NTx in serum as an indicator of bone resorption. Elevated levels of serum NTx indicate elevated bone resorption. Clinical research has demonstrated that elevated bone resorption is the primary cause of age-related bone loss and that low bone mass often results in osteopenia and is the major cause of osteoporosis.
Osteocalcin (OC) is a noncollagenous matrix protein of calcifying and calcified tissue. It is produced by osteoblasts and has been described as the most specific marker of osteoblast function. Structurally, it binds to both major bone components (collagen and apatite) and is believed to play a role in both bone resorption and mineralization. GCF OC might originate during bone formation as well as bone resorption. OC has been found in GCF from patients with periodontal disease, and increases in GCF OC concentration were associated with high rates of bone turnover.
Griffiths et al examined OC levels during orthodontic tooth movement. They collected GCF samples from the distal surfaces of the maxillary canines of 20 patients with fixed appliances during various stages of treatment. They were unable to establish a pattern in OC fluctuations in the subjects. They advocated further studies to establish whether OC GCF levels can be used as markers of bone turnover.
This study is aimed at assessment of the influence of orthodontic movement on the composition of cross-linked NTx marker and OC in the GCF during the various stages of tooth movement.
Material and methods
A sample of 20 subjects (10 male, 10 female; ages, 15-25 years) requiring orthodontic treatment was used in this study. The subjects had Angle Class I malocclusion with bimaxillary dentoalveolar protrusion and proclination with minimal or no crowding, and all required the extraction of all 4 first premolars as part of their orthodontic treatment. The treatment plan included fixed orthodontic therapy with extraction of the first premolars, followed by individual canine retraction, maximum anchorage conservation, space closure, finishing, detailing, and a fixed lingual retainer.
These subjects had no oral and systemic diseases and no periodontal pockets, and had not been on a regimen of antibiotic therapy for at least 3 months before the study. The subjects were willing to strictly adhere to the oral-hygiene instructions provided by the investigator (S.A.A.) and agreed to follow the oral-hygiene program and the orthodontic treatment prescribed for them.
All patients were treated with conventional straight wire (0.022 × 0.028 in) mechanotherapy (Discovery brackets, Dentaurum, Ispringen, Germany). The first premolar extractions were done at the start of treatment or at least 3 months before the canine retraction so that the bone remodeling from the healing socket would not influence our study. The patients were banded and bonded with a 0.022-in slot preadjusted edgewise bracket system (Discovery brackets, Dentaurum). Leveling and alignment were completed by 3 to 4 months from the start of treatment. After leveling and alignment, the retraction of the canine began on a 0.019 × 0.025-in stainless steel preformed standard arch form (archwires, 3M Unitek, Monrovia, Calif). Canine retraction was performed on one side chosen at random, with Nitinol closed-coil springs (9 mm) (3M Unitek) capable of delivering approximately 125 g of constant force. The canine on the other side acted as the control.
Thorough oral prophylaxis was done 2 weeks before the collection of samples. All patients complied with strict oral-hygiene instructions to rinse twice daily with 0.5 oz of 0.2% chlorhexidine gluconate throughout the study period and to brush their teeth at least 2 times a day with a toothbrush and toothpaste. The patients came for periodic recalls to evaluate their oral hygiene. They were instructed not to take any medications or drugs including nonsteroidal anti-inflammatory drugs during the study period.
GCF samples were collected according to the method of Lamster et al. The individual crevicular sites were isolated with cotton rolls and gently air dried. Then 6 precut methylcellulose filter strips (Periopaper strips, ProFlow, Amityville, NY) were placed in the crevice at the mesiolabial line angle, midlabial surface, distolabial line angle, distopalatal line angle, midpalatal surface, and mesiopalatal line angle until mild resistance was felt. These were left in place for 60 seconds while maintaining isolation. The 6 strips were immediately placed in individual sealed plastic tubes (Cryotube, 2.0 mL, NUNC, Roskilde, Denmark) and snap frozen at −70°C until further processing was carried out. The GCF was collected from the maxillary canines before canine retraction, 1 hour after starting canine retraction, and then on days 1, 7, 14, and 21 after canine retraction.
Levels of NTx were quantitated in samples by using a commercially available competitive-inhibition enzyme-linked immunosorbant assay (Osteomark NTx, Ostex International, Seattle, Wash). The samples were diluted with kit diluents and placed into wells coated with NTx epitope. Purified murine monoclonal antibody against NTx conjugated to horseradish peroxidase was added to each well. The plates were covered and incubated at room temperature for 90 minutes. The plates were washed, and the chromagen reagent (tetramethylbenzidine in dimethylsulfoxide with buffered hydrogen peroxide) was added to each well. The plates were then covered and incubated for 30 minutes. At the end of the incubation period, stop reagent (1 N of sulfuric acid) was added, and absorbance values were determined spectrophotometrically at 450 nm, by using a 650-nm filter, for optical density.
A standard curve was plotted by graphing absorbance vs concentrations of the standards (known concentrations of NTx that were provided in the kit) with a 4-parameter logistic curve-fitting equation. The NTx concentration was calculated by using a standard calibration curve. Assay values are reported in nanomoles of bone collagen equivalents per liter (nmol BCE/L).
An enzyme-linked immunoassay kit (Gla-type OC enzyme-linked immunoassay kit, Takara Shu-zou, Kyoto, Japan) was used for determination of Gla-type OC concentrations in the GCF samples, according to the manufacturer’s protocol. Gla-type OC contains 3 ý-carboxyglutamic acid (Gla) residues and is considered to be the active form of the molecule that can bind to calcium. The enzyme-linked immunoassay kit used in this study has been improved by using antibody precoated microtiter plates. In brief, 100 μL of test samples and bovine Gla OC standard solution were added to capture antibody (mouse monoclonal anti-Gla OC) precoated microtiter plate and incubated for 2 hours at room temperature. After the washing stage, detection antibody (mouse monoclonal anti-Gla OC) conjugated to horseradish peroxidase was applied to each well and incubated for 1 hour at room temperature. After washing, 100 μL of substrate solution, containing 3, 3′, 5, 5′ tetramethylbenzine, was added to each well. The plates were incubated for 15 minutes at room termperature, followed by application of 100 μL of stop solution. After stopping the reaction with 100 μL of 1 N sulfuric acid, the test absorbance at 450 nm was measured with a microplate reader.
Statistical analysis
All statistical analyses were performed with Instat software (version 3.05, GraphPad Software, San Diego, Calif).The NTx and OC levels at the different times were compared and analyzed with analysis of variance (ANOVA). Differences were considered significant at P values less than 0.05.
Results
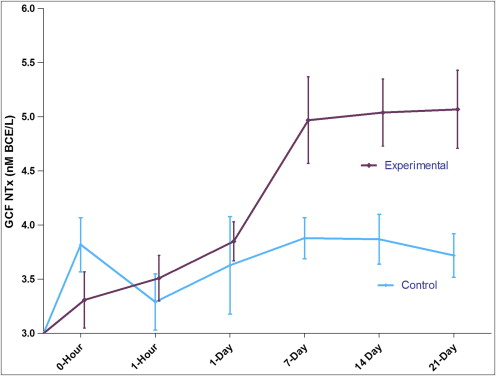
The GCF NTx level results are shown in Table I and Figure 1 . At the experiment sites, the GCF NTx level steadily increased from 3.31 ± 0.26 nmol BCE/L to 5.07 ± 0.36 nmol BCE/L. The NTx level gradually increased from day 7 to day 21. The control side did not show any statistically significant variation throughout the observation period. Even though the levels increased with time, a significant difference was observed only on the days 14 and 21, with maximum GCF NTx levels at day 21 ( P <0.01).
| Time point | Control Mean ± SEM |
Experimental Mean ± SEM |
|---|---|---|
| 0 hour | 3.82 ± 0.25 | 3.31 ± 0.26 |
| 1 hour | 3.29 ± 0.26 | 3.51 ± 0.21 |
| 1 day | 3.63 ± 0.45 | 3.85 ± 0.18 |
| 7 days | 3.88 ± 0.19 | 4.97 ± 0.40 |
| 14 days | 3.87 ± 0.23 | 5.04 ± 0.31 |
| 21 days | 3.72 ± 0.20 | 5.07 ± 0.36 |
The OC levels in the GCF showed no difference at the control site. At the experimental site where pressure was applied, the levels of OC showed higher values (3.84 ± 0.35 ng/mL) on the day 7 and onward. Significantly higher levels of OC in the GCF were observed ( P <0.01). The results are given in Table II and Figure 2 . At the control site, the OC levels did not show much variation during the various time points from hour 0 to day 21.
| Time point | Control Mean ± SEM |
Experimental Mean ± SEM |
|---|---|---|
| 0 hour | 2.37 ± 0.24 | 2.06 ± 0.11 |
| 1 hour | 2.23 ± 0.26 | 2.62 ± 0.18 |
| 1 day | 2.53 ± 0.39 | 2.70 ± 0.19 |
| 7 days | 2.31 ± 0.19 | 3.84 ± 0.35 |
| 14 days | 2.23 ± 0.22 | 4.06 ± 0.31 |
| 21 days | 2.24 ± 0.20 | 4.23 ± 0.29 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


