Oral focal mucinosis is a rare soft-tissue lesion that might result from the overproduction of hyaluronic acid by fibroblasts. The lesions are commonly found on the gingiva and palate; however, other sites, such as the tongue, have also been reported. The diagnosis of oral focal mucinosis is based on histologic analysis, and treatment involves surgical excision. Recurrences of lesions have not been reported. This article presents a patient with oral focal mucinosis that might be associated with surgically assisted rapid maxillary expansion.
Oral focal mucinosis is a rare soft-tissue lesion of unspecified etiology, first described in 1974 by Tomich, in which the connective tissue goes through focal myxoid degeneration. This soft-tissue lesion might result from the overproduction of hyaluronic acid by fibroblasts. Only 52 cases affecting patients from 4 to 74 years of age have been reported. Oral focal mucinosis is commonly found on the gingiva and palate and on the mucosa overlying the maxillary alveolar process ; however, other sites, such as the tongue, have also been reported. The oral focal mucinosis lesion is a painless, sessile, or pedunculated mass that is similar in color to the surrounding mucosa. The lesion occurs predominantly in adults during the fourth or fifth decade of life, although there are rare cases reported in children and adolescents. Oral focal mucinosis shows a 2:1 female-to-male predilection.
Histologically, oral focal mucinosis is characterized by a well-circumscribed area of myxomatous tissue containing hyaluronic acid and elongated, fusiform, or ovoid fibroblasts. Inflammatory cells may be present in the center of the lesion. The mucosa overlying the lesion and the surrounding connective tissue are unaffected. The diagnosis of oral focal mucinosis is based on histologic analysis, and treatment involves surgical excision. Recurrences of lesions have not been reported.
We present a case of oral focal mucinosis that might be associated with surgically assisted rapid maxillary expansion (RME). RME is used to treat young patients with maxillary atresia. In adults, this procedure has high failure rates because of the increased rigidity of maxillary sutures, causing dental inclinations, bone dehiscence, and gingival recession. Because of these complications, surgically assisted RME is indicated.
In our patient, the association between oral focal mucinosis and surgically assisted RME was established by the appearance of an oral focal mucinosis lesion in the region where the maxillary sutures were opened. Murray and Cleall reported that 14 days after the midpalatal suture was opened with RME in rhesus monkeys, the suture connective tissue appeared to be cellular and disorganized. This procedure applies heavy forces to open the suture, resulting in the formation of osteoid tissues. In our patient, we considered the possibility that fibroblasts stimulated by trauma from the surgically assisted RME produced hyaluronic acid, not osteoid tissues, causing the oral focal mucinosis.
Case report
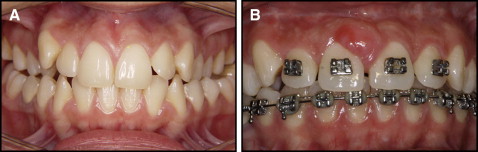
A 20-year-old white woman came to the clinic at the Faculty of Dentistry at the University of São Paulo, São Paulo, Brazil, for orthodontic preparation for orthognathic surgery. Her complaint was a bilateral posterior crossbite ( Fig 1 , A ). After the diagnosis of maxillary atresia was made, a hyrax palatal expander was placed on the first premolars and the first molars, and the patient was referred for surgically assisted RME.
A subtotal LeFort I osteotomy was used to perform the surgically assisted RME. During the procedure, the maxillary tuberosity was separated from the pterygoid plateau, and it was associated with osteotomy of the anterior region of the maxilla.
After 5 days, the patient returned to the clinic and was instructed to activate the screw in the appliance by a quarter turn in the morning and a quarter turn in the evening daily until the desired expansion was achieved.
Eight months after the surgically assisted RME procedure, the appearance of an asymptomatic neoformation on the attached gingiva of the labial surface of the maxillary central incisors was noted. The soft-tissue lesion was round, approximately 10 mm in diameter, firm, and sessile. The overlying mucosa was not ulcerated and was the same color as the surrounding mucosa, although a slight erythematous area was noted ( Fig 1 , B ). The patient was in good overall health. The intraoral examination showed good oral hygiene and periodontal health.
The maxillary central incisors had a positive pulp vitality test, and no radiographic changes were noted in the area of the lesion.
The lesion was completely removed by performing a full-thickness mucoperiosteal flap on the buccal and mesial surfaces of the maxillary right central incisor and on the mesial surface of the maxillary left central incisor under local anesthesia. Periodontal debridement was then performed, and the flap was repositioned and sutured. The extracted tissue was sent to pathology for examination.
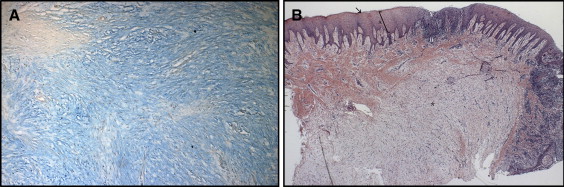
The histologic sections showed fragments of mucosa that were partially covered with hyperparakeratinized stratified squamous epithelium with the areas of acanthosis. The lamina propria consisted of dense connective tissue permeated by an intense, diffuse mononuclear inflammatory infiltrate. Throughout the connective tissue, there were areas of loose myxomatous material that were stained with alcian blue and interpreted as mucin. The histopathologic findings were consistent with the diagnosis of oral focal mucinosis ( Fig 2 ).
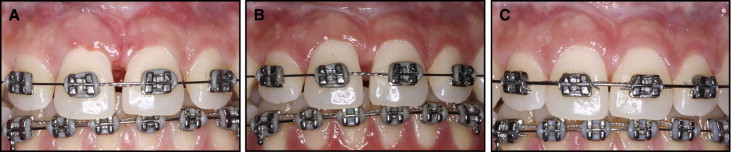
One week after surgery, the surgical site had healed without complications, and the sutures were removed ( Fig 3 , A ). The operative site was assessed 2 months and 1 year after surgery ( Fig 3 , B and C ).
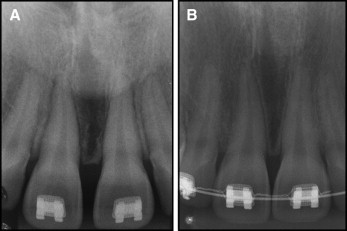
The patient was observed for 2 years after surgery, and there were no signs of recurrence. The lesion site was also monitored radiographically, and bone changes were not observed ( Fig 4 ).
Discussion
The etiology of oral focal mucinosis is unknown. Tomich described oral focal mucinosis as the counterpart to cutaneous focal mucinosis and suggested that it is caused by the overproduction of hyaluronic acid by fibroblasts during collagen production. The cause of this overproduction is unknown, and the idea that local trauma is the predisposing factor remains controversial.
Inflammatory lesions described in the literature for differential diagnoses of oral focal mucinosis include gingivitis, fibrous hyperplasia, pyogenic granuloma, peripheral giant cell granuloma, and gingival tumors such as peripheral ossifying fibroma and peripheral fibroma.
The histologic differential diagnosis for oral focal mucinosis includes soft-tissue myxoma and odontogenic myxoma. A myxoma can be an infiltrative growth, whereas focal mucinosis usually manifests as a localized area of myxomatous connective tissue. Mucin is a characteristic of many cases of focal mucinosis but is not present in myxomas. The alcian blue staining of hyaluronic acid is the predominant characteristic of oral focal mucinosis but is not characteristic of odontogenic myxomas.
The consensus for treatment of oral focal mucinosis is complete surgical excision of the lesion. Recurrence of oral focal mucinosis has not been reported in the literature ( Table ).
| Author | Year | Location | Age (y) | Sex | Duration | Recurrence after surgical excision |
|---|---|---|---|---|---|---|
| Tomich | 1974 | Palate | 40 | F | 5-10 y | No |
| Mandibular gingiva | 31 | F | 1 y | No | ||
| Mandibular gingiva | 16 | M | NA | No | ||
| Buccal mucosa | NA | F | 1 y | No | ||
| Tip of tongue | 45 | M | 2 mo | No | ||
| Mandibular alveolar mucosa | 28 | M | NA | No | ||
| Anterior hard palate | 22 | F | 4 mo | No | ||
| Anterior hard palate | 19 | F | 4 mo | No | ||
| Saito et al | 1985 | Gingiva | 35 | M | 3 mo | NA |
| Gingiva | 50 | F | NA | NA | ||
| Buchner et al | 1990 | Gingiva | 18 | F | 9 mo | No |
| Gingiva | 30 | M | 5 y | No | ||
| Gingiva | 32 | F | 1 m | No | ||
| Gingiva | 22 | F | 1 y | No | ||
| Gingiva | 53 | F | NA | No | ||
| Gingiva | 16 | F | NA | No | ||
| Gingiva | 43 | M | NA | No | ||
| Gingiva | 41 | F | NA | No | ||
| Gingiva | 37 | F | 3 y | No | ||
| Gingiva | 46 | M | 1 y | No | ||
| Mandibular retromolar area | 46 | M | 3 y | No | ||
| Alveolar mucosa | 61 | F | NA | No | ||
| Alveolar mucosa | 37 | F | NA | No | ||
| Hard palate | 38 | F | 1 y | No | ||
| Tongue | 50 | M | 2 mo | No | ||
| Gnepp et al | 1990 | Hard palate | 4 | F | NA | No |
| Soda et al | 1998 | Tongue | 68 | M | 3 y | NA |
| Etges et al | 2000 | Gingiva | 40 | F | 3 mo | NA |
| Gingiva | 43 | M | 8 y | NA | ||
| Iezzi et al | 2001 | Gingiva | 48 | M | 8 mo | No |
| Aldred et al | 2003 | Lip | 38 | F | NA | No |
| Lip | 74 | M | NA | No | ||
| Gingiva | 30 | F | 1 mo | No | ||
| Gingiva | 16 | F | 4 mo | No | ||
| Gingiva | 49 | M | 10 y | No | ||
| Gingiva | 31 | F | 6 mo | No | ||
| Gingiva | 52 | M | 1 y | No | ||
| Gingiva | 40 | F | 4 mo | No | ||
| Gingiva | 37 | F | 3 mo | No | ||
| Gingiva | 35 | F | 1 y | NA | ||
| Gingiva | 33 | F | 1 y | NA | ||
| Gingiva | 68 | M | 1 y | NA | ||
| Buccal mucosa | 56 | F | NA | NA | ||
| Mouth | 60 | F | 1 y | NA | ||
| Tongue | 55 | M | 3 mo | No | ||
| Talacko et al | 2004 | Buccal mucosa | 63 | F | NA | NA |
| Gingiva | 24 | M | NA | NA | ||
| Soares de Lima et al | 2008 | Gingiva | 36 | F | 4 mo | No |
| Gabay et al | 2010 | Gingiva | 44 | F | 3 y | NA |
| Madhusudhan et al | 2010 | Hard palate | 50 | M | 2 mo | No |
| Gingiva | 26 | F | 3 mo | No | ||
| Lee et al | 2012 | Gingiva | 17 | F | NA | NA |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


