(1)
Forensic Medicine and Clinical Toxicology, Mansoura University, Mansoura, Egypt
21.1 Introduction
21.4 Sample Collection
21.4.1 Collection Methods
21.4.2 Collection Devices
21.5.1 Preanalytical Manipulations
21.5.2 Immunological Methods
21.5.3 Chromatographic Methods
21.6.1 Pathophysiology
21.6.2 Drugs Monitored in Saliva
21.6.3 Drugs of Abuse
21.6.4 Doping Drugs
21.7.1 Advantages of Use of Saliva
21.7.2 Safety
21.7.3 Drawbacks of Use of Saliva
21.8 Teeth in Toxicology
21.8.1 Heavy Metals
21.8.2 Tobacco
21.8.4 Fluoride
21.8.5 Tetracyclines
21.8.6 Other Drugs
21.9 Conclusions
Abstract
Whole saliva is a mixture of oral fluids and includes secretions from salivary glands in addition to several constituents of nonsalivary origin (Navazash 1993). Oral fluid is an alternative biological matrix that might have advantages over urine for drug analysis in treatment programs. Whole saliva can be collected noninvasively and by individuals with limited training. In recent years, saliva has attracted much attention, in particular among people interested in the determination of drug concentrations. This suggests that saliva might be substituted for plasma in the areas of pharmacokinetic studies and drug monitoring because of the growing interest in noninvasive procedure (Vindenes et al. 2011).
21.1 Introduction
Whole saliva is a mixture of oral fluids and includes secretions from salivary glands in addition to several constituents of nonsalivary origin (Navazash 1993). Oral fluid is an alternative biological matrix that might have advantages over urine for drug analysis in treatment programs. Whole saliva can be collected noninvasively and by individuals with limited training. In recent years, saliva has attracted much attention, in particular among people interested in the determination of drug concentrations. This suggests that saliva might be substituted for plasma in the areas of pharmacokinetic studies and drug monitoring because of the growing interest in noninvasive procedure (Vindenes et al. 2011).
Oral fluid is an exciting alternative matrix for monitoring drugs of abuse in the workplace, clinical toxicology, criminal justice, and DUI (driving under the influence of drugs) programs. In 2004, the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA) proposed recommended guidelines for oral fluid collection, point-of-collection testing devices, low-cutoff concentrations, and screening and confirmation methods (Bush 2008).
Human teeth can be adversely affected by environmental chemicals, such as heavy metals and dioxin (Billings et al. 2004), and drugs, including tetracycline, anticonvulsants, and those used in chemotherapy (Alpaslan et al. 1999). Mercury in teeth amalgam was reported to become absorbed from teeth to cause systemic toxic effects (Mutter 2011).
21.2 Physiology and Biochemical Aspects
Whole saliva (mixed saliva) is a mixture of oral fluids and includes secretions from both the major and minor salivary glands in addition to several constituents of nonsalivary origin, such as gingival cervicular fluid, expectorated bronchial and nasal secretions, serum and blood derivatives from oral wounds, bacteria and bacterial products, viruses and fungi, desquamated epithelial cells, other cellular components, and food debris (Sreebny 1989).
The human salivary glands produce serous and mucinous saliva containing minerals, electrolytes, buffers, enzymes and enzyme inhibitors, growth factors and cytokines, immunoglobulins, mucins, and other glycoproteins (Tabak 1995). Mucins are a group of glycoproteins that contribute to the viscoelastic character of the mucosal secretions (Schenkels et al. 1995).
Proteins that are found in saliva, such as lactoferrin, lysozyme peroxidase, defensins, and histatins, can destroy or inhibit the growth of microorganisms in oral cavity (Xu et al. 1991; Schenkels et al. 1995). In addition, saliva has lubricating functions and aids in the digestion of food (Mandel 1987).
Saliva is alkaline with a pH between 6.4 and 7 (Navazash 1993), a specific gravity of 1.007, and a viscosity of 99.5% water and 0.5% solid contents (40% inorganic constituents/60% organic constituents). Organic constituents are mucin, enzymes, amylase, lysozyme, albumin, globulin, urea, uric acid, cholesterol, vitamins, and phospholipids (Kaufman and Lamster 2002).
Salivary composition depends on many factors: stimulation, diet, age, time of day, disease, etc. Lower pH values occur more frequently among caries-susceptible individuals. The amount of saliva secreted by an adult in 24 h varies between 1,000 and 1,500 ml. In the absence of obvious external stimuli, the rate of salivary secretion in adults is 0.1–0.25 ml/min; values <0.1 ml/min should be considered abnormal. The stimulated flow rate varies between 1 and 2 ml/min, and values <0.5 ml/min should be considered abnormal. The resting saliva reflects the basal flow rate. Stimulated saliva is present in our mouths for up to about 2 h of the day (Langel et al. 2008).
21.3 Regulation of Salivary Gland Secretions
Secretion of saliva is governed by the central nervous system along with the autonomic nervous system. Reflex salivary flow occurs at a low “resting” rate and for short periods of the day. A more intense taste or chewing stimuli evoke up to tenfold increases in salivation. All salivary glands are supplied by cholinergic parasympathetic nerves, which release acetylcholine that binds to muscarinic receptors, evoking the secretion of saliva by acinar cells. Most salivary glands also receive a variable innervation from sympathetic nerves, which release noradrenalin, causing a greater release of stored proteins (Proctor and Carpenter 2007).
Stimulated secretion occurs via nervous reflexes. Neural mechanoreceptors and chemoreceptors in the oral cavity respond to dryness of mucosa, chewing chemicals in foods, and texture of the food. Afferent impulses are integrated in the medulla, and the salivary center receives inputs from the cortex, amygdala, and hypothalamus. Salivary gland secretions may be inhibited temporarily with infections or drugs. Permanent inhibition occurs in irradiation of the head and neck (Rai 2007).
A number of drugs can affect the secretion of oral fluid. These include amphetamines, cannabis, sedating antihistamines, antipsychotic drugs, anticholinergic drugs, and a number of antidepressants. There are less commonly used drugs that increase flow, including clonidine, pilocarpine, and beta-2 stimulants (Aps and Martens 2005).
21.4 Sample Collection
21.4.1 Collection Methods
A variety of methods are available for collecting saliva. Some involve stimulating saliva production, while others target the collection of unstimulated saliva. According to Navazash (1993), unstimulated saliva can be collected by the draining method, which is performed by allowing saliva to drip from the mouth into a collection container. Several techniques may be used to collect stimulated saliva. The simplest involves tongue, cheek, or lip movements without the use of an external stimulus. Chewing paraffin wax, Parafilm, Teflon, rubber bands, gum base, or chewing gum are usually referred to as mechanical methods of stimulating saliva production. A lemon drop or citric acid can be placed in the mouth to provide a gustatory stimulus for saliva production. Following stimulation by one or more of these methods, saliva can be spit, suctioned, or swabbed from the mouth (Hold et al. 1995). The collected saliva is then put into a vial, centrifuged at 3,000 rpm for 5–10 min, and supernatant fraction is sealed following the complete chain of custody (Bosker and Huestis 2009).
There are several potential problems associated with stimulating saliva production. Parafilm absorbs some drugs and therefore gives erroneous results when saliva is tested for drugs or drug metabolites. Also, paraffin contains compounds that may affect chromatographic analyses—again affecting drug testing accuracy. Some salivary stimulants may change the salivary composition and, therefore, affect the saliva-drug concentration. For example, citric acid may change saliva pH and consequently alter drug concentrations in the saliva. Citric acid and cotton have also been shown to alter immunoassay drug test results (Hold et al. 1995; Gallardo and Queiroz 2008).
21.4.2 Collection Devices
Oral fluid-collection devices are now used for large-scale testing applications. Many devices collect 1 ml of oral fluid. They have names such as Oral Diffusion Sink and Salivette (Shipley et al. 1992), Proflow (Sonesson et al. 2008), and Orasure (Fritch et al. 2011) (Figs. 21.1 and 21.2).
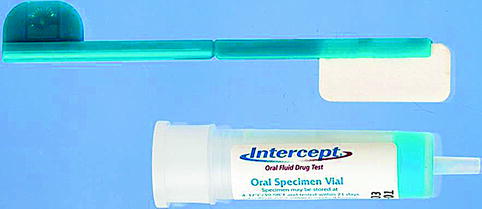
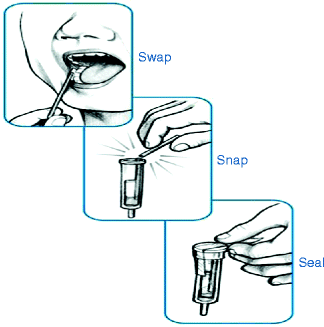

Fig. 21.1
An example of an oral fluid device consisting of a swab and a collecting vial

Fig. 21.2
The method of oral fluid collection using the Intercept collecting device
Some oral fluid-collection devices have built-in volume adequacy indicators, facilitating appropriate collection, whereas other manufacturers report only approximate amounts collected. Another approach is gravimetric determination by weighing the device before and after oral fluid collection (Dickson et al. 2007).
21.5 Analytical Methodology for the Measurement of Drugs in Saliva
21.5.1 Preanalytical Manipulations
Once samples have been collected, it is important that they be properly stored (at −20 °C or −80 °C) unless analyses are to be performed immediately. Long-term storage of salivary samples at room temperature requires cortisol (Cone and Weddington 1989).
Most authors use the centrifugate, either by extracting the centrifugate with an organic solvent at a desirable pH or by using the centrifugate directly in the analytical process without sample pretreatment (Hold et al. 1995).
21.5.2 Immunological Methods
Immunological methods of analysis have been widely used for monitoring drugs in saliva and other body fluids, mainly because of their relative simplicity of use, requiring little or no extractive operations, their application to large batch analyses, and their sensitivity (Caddy 1984). However, they do not always possess the specificity required for distinguishing metabolites from the parent drug (Paxton and Donald 1980).
Radioimmunoassay (RIA) techniques have been used especially for the analysis of hormones in saliva and some drugs as cocaine, cannabinoids, haloperidol, theophylline, and cotinine. An enzyme-multiplied immunoassay technique (EMIT) may be used, but it is less sensitive (Hold et al. 1995).
21.5.3 Chromatographic Methods
Thin-layer chromatography (TLC) has the advantage of simplicity and allows the simultaneous determination of several samples. However, the major limiting factor is the detection of the respective spots on the thin-layer plate at low drug levels in saliva (Hold et al. 1995).
After the initial screen test, confirm suspect positive test results by gas chromatography/mass spectrometry (GC/MS) or liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS). These confirmation methods provide the most sensitive “fingerprint” identification available for the target drug. LC-MS/MS permits the simultaneous analysis of multiple, nonvolatile, labile, polar, and/or high-molecular-weight compounds in a limited oral fluid volume (Samyn et al. 2007).
21.6 The Use of Saliva for Drug Monitoring
21.6.1 Pathophysiology
There are several ways by which serum constituents that are not part of the normal salivary constituents (i.e., drugs and hormones) can reach saliva. Within the salivary glands, transfer mechanisms include intracellular and extracellular routes. The most common intracellular route is passive diffusion (across a concentration gradient), although active transport has also been reported. Ultrafiltration, which occurs through the tight junctions between the cells, is the most common extracellular route (Haeckel and Hanecke 1993).
A fundamental prerequisite for this diagnostic application of saliva is a definable relationship between the concentration of a therapeutic drug in blood (serum) and the concentration in saliva. The presence of a drug in saliva is influenced by the physicochemical characteristics of the drug molecule and its interaction with the cells and tissues of the salivary glands, as well as by extravascular drug metabolism. Factors such as molecular size, lipid solubility, and the degree of ionization of the drug molecule, as well as the effect of salivary pH and the degree of protein binding of the drug, are important determinants of drug availability in saliva (Siegel 1993).
Generally, smaller molecules diffuse more easily than larger ones and lipophilic molecules diffuse more easily than lipophobic molecules (Haeckel and Hanecke 1996). Drugs that are not ionizable, or are not ionized within the pH range of saliva, are the most suited to salivary drug monitoring. The unbound (usually the pharmacologically active) fraction of the drug in serum is available for diffusion into saliva (Haeckel 1993).
Weak bases are detected in higher concentrations and for longer times in oral fluid than in plasma because of oral fluid ion trapping, as blood pH is 7.4 and that of oral fluid is 4–6 (Bosker and Huestis 2009).
21.6.2 Drugs Monitored in Saliva
Anticonvulsant drugs can be easily monitored using saliva, including phenobarbital, phenytoin, carbamazepine, lamotrigine, oxcarbazepine, topiramate, levetiracetam, and gabapentin, providing an approximation of the serum-free level. For highly protein-bound medications such as phenobarbital, phenytoin, and carbamazepine, saliva has the advantage of providing an approximation of the serum-free, active moiety level (Baumann 2007). Carbamazepine levels were found to be 38% of serum carbamazepine levels (Rosenthal et al. 1995). In another study, salivary levels of phenobarbital and phenytoin demonstrated excellent correlations (r = 0.98 and 0.97, respectively) with their serum levels (Kankirawatana 1999).
Saliva may be used for monitoring patient compliance with psychiatric medications (El-Guebaly et al. 1981). A significant correlation (r = 0.87) exists between the salivary and serum lithium levels in patients receiving lithium therapy (Ben-Aryeh et al. 1980, 1984). A lower correlation (r = 0.68) was found between salivary and total serum levels of cyclosporine. Salivary levels of this drug reflect the serum levels of free cyclosporine. Therefore, salivary cyclosporine levels may correlate better with serum levels of free, rather than total, cyclosporine (Coates et al. 1988).
Similarly, the salivary theophylline concentration demonstrated a better correlation with the serum concentration of free theophylline (r = 0.85) than with the serum concentration of total theophylline (r = 0.85) (Kirk et al. 1994). Stimulated saliva predicted both the total and unbound serum theophylline concentrations within ±1 mcg/ml in 62.5% and 92.9%, respectively, of the samples examined. The therapeutic range for saliva, which corresponds to the accepted total serum concentration range of 10–20 mg/l, was found to be approximately 5.6–11.3 mg/l (Blanchard et al. 1991).
Van Oosten et al. (1986) detected metronidazole in saliva. Measuring chloroquine concentrations, the saliva-to-total plasma concentration ratio was found to be approximately constant (Brown-Awala et al. 1989). Also, saliva may also be used for monitoring levels of anticancer drugs such as irinotecan (Takahashi et al. 1997). Paraquat has been detected in oral fluids after exposure to acute polychlorinated biphenyls (PCBs) and polychlorinated-dibenzo-p-dioxins (Ogawa et al. 2003).
21.6.3 Drugs of Abuse
Following drug use, the appearance of the drug in saliva follows a time course that is similar to that of serum. In contrast, drugs appear at a later time point in urine. The presence of illicit drugs, and not their concentration, is usually sufficient for forensic purposes (Kaufman and Lamster 2002). The LC-MS/MS method has been used for screening the most commonly abused drugs and their metabolites in preserved oral fluid to detect a relatively recent use of drugs (Badawi et al. 2009; Vindenes et al. 2011).
The salivary ethanol concentration may be used as an index of the blood ethanol concentration, provided that the salivary sample is obtained at least 20 min following ingestion. This will allow for the absorption and distribution of alcohol and prevent a falsely elevated reading due to the oral route of consumption (McColl et al. 1979). The saliva-to-serum ratio of ethanol is generally about 1 (Penttila et al. 1990).
Oral fluid testing is useful for monitoring codeine exposure, with a detection window of 7–21 h for single doses, depending on cutoff concentrations (Kim et al. 2002).
Other drugs can be identified in saliva, such as amphetamines, barbiturates, benzodiazepines, phenyclidine, cocaine, and opioids (Kidwell et al. 1998; Øiestad et al. 2007). Also, hypnotics, sedatives, and a muscle relaxant were identified (Gjerde et al. 2011). Saliva can be used to detect recent marijuana use for at least 4 h after marijuana is smoked (Isutsu et al. 1981) by means of radio immunoassay (Gross et al. 1985), ultraperformance LC-MS/MS (Badawi et al. 2009), and enzyme immunoassay (ELISA) (Schwope et al. 2010).
Saliva can be used to monitor tobacco smoking and exposure to tobacco smoke. Salivary cotinine levels were found to be indicative of active and passive smoking (Repace et al. 1998).
21.6.4 Doping Drugs
The most frequently used biological specimen for the determination of drugs in doping control is urine, since only a noninvasively obtained sample is acceptable for routine collection. Yet, even the acceptability of a urine sample is being disputed in view of the potential invasion of privacy, especially if a directly observed collection is advisable to prevent adulteration or substitution of sample (Schramm et al. 1992).
That happens, for instance, when athletes try to escape detection by using urine from someone else. Another major disadvantage of urine is the variability in the renal clearance of drugs and their metabolites, which is largely due to fluctuations in the flow rate and pH of urine. Not all drugs are excreted in the urine; for instance, the lipid-soluble β-blocking drugs tend to be rapidly eliminated by various metabolism systems in the liver (McDevitt 1987). At present, saliva is not used as a biological fluid for doping control (Rai 2007).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


