Key points
- •
Review the epidemiology, etiologic risk factors, clinical presentation, recognition, and diagnosis of oral precancer and cancer.
- •
Recommendations on clinical examination and early diagnostic techniques (including adjuncts) are presented.
- •
Treatment and complications from treatment of oral cancer are only briefly discussed.
Cancer is the second leading cause of death in the United States. In 2013, more than 580,000 people died of cancer, from almost 1.7 million new cases. Oral and pharyngeal cancers account for nearly 40,000 cases of cancer (incidence of 10 per 100,000) and approximately 8000 deaths per year in the United States and is the sixth most common cancer worldwide ( Table 1 ). Oral and pharyngeal cancer is more common than cervical or liver cancer, Hodgkin lymphoma, and brain, stomach, or ovarian cancer. More than 90% of these oral and pharyngeal cancers are squamous cell carcinomas (SCCs). The other 10% comprise salivary gland tumors, lymphoma, sarcoma, and others. The 5-year survival rate from these cancers has improved only modestly in the past 30 years and remains at approximately 55% to 60%. Head and neck cancer is staged by the TNM classification system and directly related to survival. The incidence and survival are also dependent on the anatomic site based on TNM staging ( Table 2 ). The 5-year survival rate for Whites is approximately 58%, whereas that for Blacks is only 31%.
| Location | Incidence | % | % of All Cancer |
|---|---|---|---|
| Oral | 17,900 | 48 | 2.4 |
| Oropharynx | 3500 | 10 | 0.8 |
| Nasopharynx | 2500 | 9 | 0.5 |
| Larynx | 9800 | 25 | 1.4 |
| Maxillary sinus | 1100 | 3 | 0.2 |
| Nose | 400 | 1 | 0.1 |
| Esophagus | 800 | 2 | 0.2 |
| Ear | 300 | 1 | 0.2 |
| Site | Localized | Nodal Involvement | Distant Metastasis | Total |
|---|---|---|---|---|
| Lip | 89 | 57 | 40 | 86 |
| Floor of mouth | 65 | 31 | 14 | 44 |
| Others | 61 | 29 | 18 | 44 |
| Tongue | 52 | 22 | 7 | 33 |
The ratio of males to females diagnosed with oral and pharyngeal cancer is 2:1 over a lifetime, although the ratio comes closer to 1:1 with advancing age. Human papillomavirus (HPV)-associated oropharyngeal cancers (ie, cancers predominantly involving the palatine and lingual tonsils/base of tongue) are occurring at the most rapid rate of all head and neck cancers. Approximately 90% of oral and pharyngeal cancer is diagnosed in persons older than 40 years, and more than 50% of all cancers occur in persons older than 65 years. The average age at the time of diagnosis is 63 years. However, evidence indicates that both oral cavity and oropharyngeal cancers are occurring more frequently in younger persons (<40 years). The overall incidence of oral and pharyngeal cancers has remained stable, relative to the occurrence of newly diagnosed cancers of all sites, with absolute numbers only slightly increasing each year.
The observation that oral and pharyngeal cancer generally occurs with advancing age indicates that over time, certain sequenced alterations in the biochemical-biophysical processes (nuclear, enzymatic, metabolic, immunologic) of aging cells that have a particular genetic predisposition undergo and accumulate mutations, resulting in carcinogenic transformation. These carcinogenic changes may be influenced by oncogenes, carcinogens, and mutations caused by chemicals, viruses, irradiation, drugs, hormones, nutrients, or physical irritants.
An imbalance between abnormal cell proliferation or apoptosis (programmed cell death) may be modified by factors that alter cellular production of growth and suppressor proteins.
Malignancies of the oral cavity often begin as preneoplastic lesions in the form of lesions such as leukoplakia, erythroplakias, and erythroleukoplakia. Leukoplakia is associated with tobacco and alcohol use and chronic inflammation with the risk of malignant transformation to SCC of approximately 5% to 17% ( Fig. 1 ). Alterations in host immunity, inflammation, angiogenesis, and metabolism have been noted to be prominent clinical features in oral cancer. These tumor-induced T-lymphocyte, granulocyte, and neoangiogenesis responses in the local tumor microenvironment have been associated with increased tumor growth and metastasis and decreased survival rates. Pathologic changes in systemic responses have also been observed, including induction of antibody and other acute phase inflammatory protein responses.
This article reviews the epidemiology, etiologic risk factors, clinical presentation, recognition, and diagnosis of oral precancer and cancer. The treatment and complications from treatment of oral cancer are only briefly discussed, because complications of therapy are discussed elsewhere in this issue in the article on chemotherapy or radiation-induced oral mucositis by Lalla and colleagues ( Box 1 ).
| T: primary tumor a,b | |||
| TNM | FIGO (International Federation of Gynecology and Obstetrics) | ||
| TX | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ | ||
| T1 | Tumor ≤2 cm in greatest dimension | ||
| T2 | Tumor >2 cm but not >4 cm in greatest dimension | ||
| T3 | Tumor >4 cm in greatest dimension | ||
| T4a (lip) | Tumor invades through cortical bone, inferior alveolar nerve, floor of mouth, or skin (chin or nose) | ||
| T4a (oral cavity) | Tumor invades through cortical bone, into deep/extrinsic muscle of tongue (genioglossus, hyoglossus, palatoglossus, and styloglossus), maxillary sinus, or skin of face | ||
| T4b (lip and oral cavity) | Tumor invades masticator space, pterygoid plates, or skull base; or encases internal carotid artery | ||
| Note: superficial erosion alone of bone/tooth socket by gingival primary is not sufficient to classify a tumor as T4 | |||
| N: regional lymph nodes c | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension | ||
| N2 | Metastasis as specified in N2a, 2b, 2c below | ||
| N2a | Metastasis in a single ipsilateral lymph node, >3 cm but not >6 cm in greatest dimension | ||
| N2b | Metastasis in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension | ||
| N2c | Metastasis in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension | ||
| N3 | Metastasis in a lymph node >6 cm in greatest dimension | ||
| Note: midline nodes are considered ipsilateral nodes | |||
| M: distant metastasis | |||
| MX | Distant metastasis cannot be assessed | ||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| Stage grouping | |||
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T1, T2 | N1 | M0 |
| T3 | N0, N1 | M0 | |
| Stage IVA | T1, T2, T3 | N2 | M0 |
| T4a | N0, N1, N2 | M0 | |
| Stage IVB | Any T | N3 | M0 |
| T4b | Any N | M0 | |
| Stage IVC | Any T | Any N | M1 |
a 947,2418.
b A help desk for specific questions about the TNM classification is available at http://www.uicc.org/index.php?id=508 .
c The regional lymph nodes are the cervical nodes.
Anatomic sites
The tongue is the most common site for oral cancer in both American men and women ( Table 3 ). Recent data (2011) indicate that about 46% (12,000:26,100) of all oral cavity cancers (excluding the pharynx) occur on the tongue. There are some differences in the most common oral and pharyngeal sites in other areas of the world, such as Southeast Asia, where nasopharyngeal cancer is more common, and in India, where buccal mucosa carcinomas are the most common oral sites. Data from the SEER (Surveillance Epidemiology and End Results) program show that more than 37% of all oral cancers diagnosed in the United States between 1985 and 2011 occurred in the tongue, followed by the lip and floor of the mouth. More recently, the incidence of tongue malignancies has increased to account for 46% of oral cancers. The other oral anatomic sites in decreasing order are: lip (18%), floor of mouth (12%), salivary glands (10%), buccal mucosa (5%), gingival (5%), and palate (4%).
| Oral Location | Incidence | % |
|---|---|---|
| Tongue | 12,000 | 46 |
| Lip | 4200 | 18 |
| Floor of mouth | 2300 | 12 |
| Salivary | 2000 | 10 |
| Buccal | 1100 | 5 |
| Gingiva | 1100 | 5 |
| Palatal | 800 | 4 |
Anatomic sites
The tongue is the most common site for oral cancer in both American men and women ( Table 3 ). Recent data (2011) indicate that about 46% (12,000:26,100) of all oral cavity cancers (excluding the pharynx) occur on the tongue. There are some differences in the most common oral and pharyngeal sites in other areas of the world, such as Southeast Asia, where nasopharyngeal cancer is more common, and in India, where buccal mucosa carcinomas are the most common oral sites. Data from the SEER (Surveillance Epidemiology and End Results) program show that more than 37% of all oral cancers diagnosed in the United States between 1985 and 2011 occurred in the tongue, followed by the lip and floor of the mouth. More recently, the incidence of tongue malignancies has increased to account for 46% of oral cancers. The other oral anatomic sites in decreasing order are: lip (18%), floor of mouth (12%), salivary glands (10%), buccal mucosa (5%), gingival (5%), and palate (4%).
| Oral Location | Incidence | % |
|---|---|---|
| Tongue | 12,000 | 46 |
| Lip | 4200 | 18 |
| Floor of mouth | 2300 | 12 |
| Salivary | 2000 | 10 |
| Buccal | 1100 | 5 |
| Gingiva | 1100 | 5 |
| Palatal | 800 | 4 |
Stage at diagnosis and survival
Approximately half of all patients with oral and pharyngeal cancers survive their disease 5 years after treatment. Theses figures are less favorable for SCC of the tongue (33%).
In the United States, the outcomes are more favorable for Whites than for Blacks (58% vs 31%; 5-year survival rates). Genetics is significantly involved in the predisposition to cancer, but socioeconomic status, education, and access to the health care system also have an influence. The survival rates for advanced tumors are lower compared with earlier detected, localized cancers. At the time of diagnosis, nearly 50% of all carcinomas of the tongue have already metastasized. An additional 35% to 40% metastasize within 5 years. If all cases of oral cancer were diagnosed and treated early as localized tumors, almost 80% of all patients would survive 5 years. Little progress has been made during the past 40 years in regard to early diagnosis. In addition, based on more than 25,000 SEER program oral/pharyngeal cases for which there was adequate information, localized/early oral cancers were outnumbered by advanced tumors 59% to 41%.
The lip was the only major site at which localized cancers were more frequently found than more advanced cancers. Advances in the treatment of oral and pharyngeal cancer have not led to significantly improved survival, and therefore, earlier diagnosis is the most important factor in improving oral and pharyngeal cancer control and reducing morbidity and mortality.
TNM staging
Head and neck cancer is staged by the TNM classification (see Table 2 ). The TNM classification is based on tumor size, regional lymph node involvement, and distant metastasis. The classification is based on imaging, including computed tomography (CT), magnetic resonance imaging (MRI), and sometimes positron emission tomography (PET) scans are used to facilitate staging, particularly of advanced disease. Most often, a chest radiograph is used to determine metastasis to the lungs. A PET scan is also useful for the identification of metastases. As an example, a patient who presents with a suspicious oral lesion, which is 2.5 cm with ipsilateral lymphadenopathy of 2 cm and a negative chest radiograph. is classified as T2N1M0. This classification is useful not only for determining the severity and prognosis of the cancer but the choice of therapy (excisional surgery, modified or radical lymph node dissection, radiation, or chemotherapy).
Cause and risk factors
The cause of oral cancer is multifactorial and involves many alterations in host immunity and metabolism, angiogenesis, exposure to chronic inflammation, in a genetically susceptible individual. The carcinogenic changes may be influenced by oncogenes, carcinogens and mutations caused by chemicals, viruses, irradiation, drugs (tobacco and alcohol), hormones, nutrients, or physical irritants.
Immune system
Multiple studies have shown that the risks of cancer increase in individuals whose immune systems are either congenitally defective or have been suppressed or altered by disease or medications. Furthermore, immune competence and immune cell surveillance diminish with age. This factor likely contributes to the association between age and malignancy.
Tobacco
Smoking tobacco is a worldwide epidemic, contributing to serious health problems and systemic diseases of immense proportions. Reports from the US Surgeon General and others conclude that cigarette smoking is the main cause of cancer mortality in the United States, contributing to an estimated 30% of all cancer deaths and substantially to cancers of the head and neck.
The association between cigarette use and oral carcinoma has been firmly established from epidemiologic studies, showing that there are more than twice as many smokers among patients who have oral cancer as among control populations.
One study found that 72% of more than 400 patients with oral cancer were smokers and 58% smoked more than 1 pack daily, showing the high risk for tobacco users.
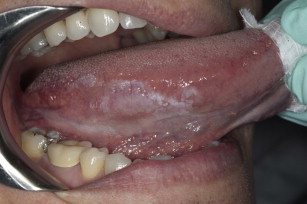
Tobacco use also increases the already high risk for developing recurrences of oral cancer as well as second primary oral and pharyngeal cancers. Smoking tobacco is the leading contributor to oral and pharyngeal cancer, but the use of smokeless tobacco has been associated as well (see Fig. 1 ).
Certain hydrocarbons isolated from tobacco products have been shown to induce carcinomas in animals under certain experimental conditions.
Benzo[a]pyrene, one of the most potent of these carcinogens, binds to nucleoproteins and is mutagenic as well as carcinogenic. The association between tobacco use and oral malignancies also seems to include cigars, pipes, and smokeless preparations.
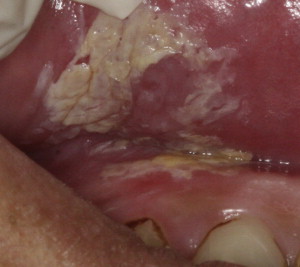
Alcohol intake has also been associated with the incidence of oral cancer, especially with long-term excessive use. One group of investigators found that 44% of 108 patients with cancer of the tongue and 59% of 68 patients with cancer of the floor of the mouth, palate, or tonsillar fossa had unequivocal evidence of alcoholic cirrhosis. Fig. 2 shows a leukoplakia on the lateral ventral tongue of a patient with a history of heavy tobacco and alcohol use. Approximately 75% drank alcohol excessively. The combined effects of tobacco and alcohol are shown in another study of more than 350 patients who had oral cancer and a mortality of 31% within 5 years.
However, definitive associations between alcohol-containing mouth rinses and the development of oral cancer have not been established. Areca nut products are another established risk factor for oral cancer.
Oral lichen planus
Oral lichen planus (OLP) is a complex, chronic, inflammatory disease. The cause of OLP is unknown, but it is believed to be an immunopathologic disease. OLP can occur in any oral site, with the buccal mucosa as the most common location. OLP can usually be recognized by the unique clinical features of reticular, annular, or punctate keratotic (white) patterns on the mucosal surface. Diagnosis should always be confirmed by biopsy.
A summary of the results from several studies performed in 7 different countries since 1981 indicates that from 0.4% to 5.6% of OLP lesions transformed to SCC.
Nutrition
Although some studies indicate a potential association with dietary factors and cancer in general, no clear dietary characteristics (deficiencies or excesses of nutrients) have been recognized that directly correlate with cancer of the oral cavity. Some recent meta-analyses have indicated a positive protective effect with a diet high in fresh fruits and vegetables.
Viruses
The role of viruses in development of oral cancer has been a matter of investigation for a long time. Those having oncogenic potential are from 2 groups: the herpesviruses and the Human papilloma viruses.
One of the most common virus groups in the world affecting the skin and mucosal areas of the body is HPV. More than 120 different subtypes of HPV have been identified, and different types are known to infect different parts of the body. The most visible forms of the virus produce warts (verruca vulgaris) on the hands, arms, legs, and other areas of the skin. Most HPVs of this type are common, harmless, nononcogenic, and easily treatable. There are other forms of HPV, which are sexually transmitted, and some of these can cause cancer. The most common of these oncogenic subtypes is HPV-16, and less common subtypes are HPV-18, HPV-31, and HPV-45.
HPV-16 causes oropharyngeal cancer. In the oral environment, HPV-16 manifests itself primarily in the oropharynx, including the base of the tongue (lingual tonsils) and palatine tonsils. These oncogenic or cancer-causing versions of HPV are also responsible for other SCCs, particularly of the cervix, anus, and penis.
HPV is covered in more detail in another article elsewhere in this issue.
A study conducted by the Johns Hopkins Oncology Center by Dr Maura Gillison furthered the premise that HPV might be associated with oropharyngeal maligancies. This study evaluated 253 patients diagnosed with head and neck cancers. In 25% of these cases, the tissue taken from tumors was HPV positive. HPV-16 was present in 90% of the HPV-positive tissues. We now know that there is a survival advantage that patients with HPV-positive oropharyngeal cancer have over patients who develop cancer from other causes (ie, tobacco). HPV-positive tumors are more susceptible and vulnerable to the radiation treatments than their tobacco-induced counterparts. It is possible that in the future, clinical trials will be conducted that will establish some different treatment protocol for HPV-positive oral cancers (see article elsewhere in this issue by Pringle).
Clinical assessment
Clinicians must perform a comprehensive history and physical examination on every patient. The standard of care examination includes not only thorough examination of every intraoral mucosal surface but the extraoral head and neck tissues, including lymph nodes, as well (described in detail later; also see http://www.dentalce.umn.edu/OralCancerVideo/home.html ).
The detection of any abnormal finding requires further investigation contingent on the nature of the abnormality and the experience of the clinician. Regardless of ability, the clinician has an obligation to inform the patient, in terms they understand, about the nature of an abnormal examination finding and management recommendations. Any persistent and progressive epithelial lesion without any identifiable cause should raise suspicion and must be evaluated to rule out premalignant or malignant changes.
Signs and symptoms
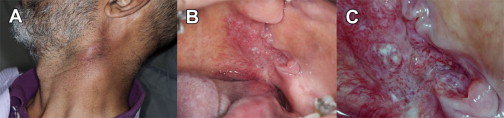
Oral cancers and premalignant oral lesions can have variable clinical presentations. At one end of the disease spectrum, the signs and symptoms of advanced cancers are generally ominous. Such cancers are often large, grossly exophytic, or deeply ulcerated, they show induration, they bleed easily on slight provocation, and have metastasized to regional lymph nodes, which is associated with palpable lymphadenopathy ( Fig. 3 : advanced oral cancer). Because the cancer has infiltrated deeper structures, including nerves, muscles, and even bone, patients may experience significant pain, have difficulty chewing, swallowing, or even speaking. At the other end of the disease spectrum, early cancers and premalignant oral lesions have more subtle signs and often do not elicit any detectable symptoms. Their early detection is contingent on a careful visual and tactile examination, in which the clinician must interpret the features of an abnormal examination finding(s), such as lesion color, lesion number and size, lesion topography or morphology, or evidence of induration suggesting submucosal infiltration (in the case of a malignancy), to assess the risk of suspicion for lesions with malignant potential.
Most epithelial lesions are benign, and many may be diagnosed based on their clinical features alone, without the need for further investigation. Frictional keratoses (white lesions caused by chronic low-grade friction to the mucosa) are commonly encountered, and if the putative frictional source is removed (eg, smoothing off a sharp tooth), such lesions should show signs of resolution in 2 to 3 weeks.
For those epithelial lesions for which the clinician cannot ascertain the cause, and there is suspicion for malignancy, further investigation is warranted, such as a biopsy with histopathologic examination. The decision of referring a patient to a specialist for biopsy of a lesion that turns out to be benign versus not referring a patient with a lesion that turns out to be an early cancer can be difficult, particularly for clinicians with minimal experience in mucosal pathology.
The term potentially malignant disorders (PMDs) has been ascribed to epithelial lesions for which there is no apparent cause and therefore should undergo further evaluation to rule out malignancy or precancer. The terms leukoplakia (see Fig. 2 ), erythroplakia, and erythroleukoplakia (see Fig. 3 ; Fig. 4 ) are attributed to PMDs as clinical diagnoses for white patches that cannot be wiped off, red patches, or mixed red/white patches (respectively) for which there is no apparent cause and for which histopathologic examination is required to rule out malignancy or precancer.
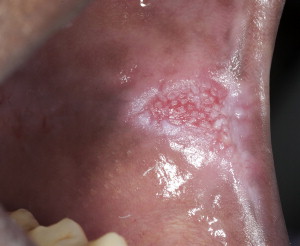
Lesions with a red component (ie, erythroplakia and erythroleukoplakia: Fig. 4 ) are more likely to represent malignancy or precancer than a white-only lesion (leukoplakia), although there are exceptions. In addition to lesion color, other clinical features are important. Large lesions (>2 cm in diameter) or multifocal lesions should heighten suspicion. Proliferative verrucous leukoplakia (PVL) is a clinical term describing patients with multifocal PMDs who have a high risk for malignant transformation. In terms of topography and morphology, PMDs can present as homogeneous and relatively flat, plaquelike lesions (generally these are white), to nonhomogeneous and thicker lesions with variable topography described as fissured, granular, nodular, or even verrucous or wartlike (such lesions may be white or mixed red-white lesions). Ulceration is defined as a loss of epithelium and may be commensurate with PMDs as a sign of malignancy or in conjunction with high-grade dysplastic lesions (see section on dysplasia), in which the epithelium is lost after local trauma. Ulcers may present alone, or with mixed red-white PMDs. Other clinical features include the presence of induration, an ominous feature associated with palpable firmness associated with submucosal extension, and friability, a feature associated with abnormal bleeding on slight provocation, suggestive of increased angiogenesis.
It is important for clinicians to be aware that although patients with small early stage cancers are often asymptomatic, the presence of pain is not predicated on tumor size and a mass effect on local nerves, but rather likely to be related to sensitization of nerves secondary to the release of pain mediators from the cancer. The pain may be elicited only during oral function (a mechanical allodynia) or when palpating the lesion. Spontaneous pain is also possible and predicts a higher likelihood of metastatic spread.
Clinicians must also recognize that patients can initially present complaining of a swelling in the neck, which represents lymph node metastasis, yet the primary cancer site may be completely asymptomatic. Generally, when there is lymphadenopathy associated with metastatic spread from an oral cavity primary cancer, the cancer is usually visualized during the examination. However, with the recent increase in the number of HPV-associated oropharyngeal cancers, tonsillar primary cancers can be small, asymptomatic, and may not be visualized during the examination or on imaging by CT or MRI (hence the term unknown primary).
The following are clinical diagnoses/common presenting signs of PMDs or oral carcinoma :
- •
Leukoplakia: white patches of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer; a homogeneous leukoplakia tends to be smooth, uniformly thin and consistent in whiteness, often with shallow fissure; a nonhomogeneous leukoplakia tends to have surface texture and has variable thickness (see Fig. 2 )
- •
Erythroplakia: a fiery red patch that cannot be characterized clinically as any other definable disease
- •
Erythroleukoplakia: a mixed red and white patch that cannot be characterized clinically as any other definable disease (see Fig. 4 )
- •
Ulceration or erosion that cannot be characterized clinically as any other definable disease; ulceration may be solitary or part of a white, red or mixed red/white patch
- •
Induration: mucosal firmness or hardness caused by submucosal infiltration of a carcinoma
- •
Fixation: invasion of carcinoma into deeper tissues
- •
Chronicity/persistence: failure of lesions to heal; cancer is not a spontaneously reversible disease; therefore, a malignant lesion normally does not disappear in the absence of definitive antitumor therapy
- •
Lymphadenopathy: enlargement of regional nodes caused by metastatic spread of neoplastic cells through lymphatic vessels; nodes are usually painless and often become fixed because of capsular erosion and local infiltration; tumors that involve marked induration, fixation, and lymphadenopathy are signs of advanced cancer
It is critical for clinicians to remember that if their clinical impression of a lesion is benign and they treat the lesion empirically (eg, smooth off a sharp cusp in the case of a lesion with possible frictional/traumatic cause), they must closely follow the patient to resolution. Typically, in the early stages, oral carcinomas may appear as small, innocuous, harmless, minor mucosal changes, to which the unsuspecting clinician may not aggressively respond. An extended period beyond 2 to 3 weeks of watching and empirical treatments may allow the lesion to expand and potentially metastasize.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


