Obstructive sleep apnea (OSA) and obstructive sleep apnea syndrome (OSAS), apneas with clinical symptoms, are potentially fatal diseases that have a myriad of causes. The two main types of sleep apnea are obstructive and central (i.e., apnea caused by centrally induced respiratory depression and not due to obstruction), although a combination of the two is common. Surgeons are generally called upon to manage the OSA subset, because there is no good surgical option for central sleep apnea.
Understanding that the source and level of obstruction causing OSA can occur anywhere in the airway from the tip of the nose to the vocal cords, surgical management of OSAS is predicated on finding the source of anatomic constriction and either surgically dilating it or at least preventing it from collapsing or constricting during sleep. Once the diagnosis has been made using clinical history, physical examination, diagnostic nasopharyngoscopy, and objective polysomnography, a treatment plan is constructed to treat one or more of these areas of the airway.
Surgical management of OSAS has historically been divided into a group of clinical phases. This is based on the theory that one should start with the lowest morbidity procedures (phase I) and work up to more major surgery (phase II) as necessary, based on the response of the simpler procedures. Although this does make some sense, it is evident now that these “simpler” procedures are also not likely to cure patients with high levels of apnea. Therefore many surgeons now proceed immediately to more major and clinically effective phase II surgery when the severity of the clinical situation warrants it (i.e., maxillomandibular advancement in patients with high level of disease). Phase II procedures generally affect multiple areas over a larger cross section of the airway at one time and represent the best opportunity to effectively treat the disease process occurring throughout the airway. In patients with a lower apnea-hypopnea index (AHI), however, the use of phase I treatments (those that target an isolated area) are commonly still used, often in a stepwise fashion, until the OSAS is controlled or phase II treatment is indicated. Of course, this is based on the assumption that the targeted area is, in fact, the anatomic area of obstruction within the airway. With the advent of three-dimensional imaging (and hopefully with increasing use of more accurate dynamic imaging while the patient is sedated), it may be possible in the near future to better predict what treatment will have the greatest effect with the least morbidity.
Phase I treatment options are quite varied, and the choice of which one or ones to use is based on the anatomic source suspected by the clinician. The choice of surgical procedures is often also affected by the training, comfort, and experience of the surgeon. An ear, nose, and throat (ENT) surgeon might consider an early septoplasty, whereas an oral and maxillofacial surgeon (OMFS) might choose a genial osteotomy in the same patient based on their comfort in treating these anatomic areas, the medical history, and anesthetic risk of the patient.
The purpose of this chapter is to discuss the various options available for phase I treatment. With phase I treatment, as with any therapy, the most conservative approach yielding the highest increase in oxygenation and reduction of the AHI should always be considered.
Weight Loss
Although not universal, the vast majority of patients with OSA are obese, and there is a direct correlation between this disease and body mass index (BMI). The BMI is defined as the weight in kilograms divided by the height in meters squared (kg/m 2 ). A BMI of more than 25 kg/m 2 is defined as overweight, whereas a BMI of more than 30 kg/m 2 is considered obese. Not only does obesity lead to adverse systemic conditions, such as cardiovascular disease, diabetes, and certain cancers, mechanically the increased weight in the cervical region increases the likelihood of obstruction. With weight gain, there is a subsequent increase in neck circumference, which constricts the hypopharynx and oropharynx. Increased fatty deposits in the peripharyngeal area also leads to constriction of the airway. It is generally recognized that any male patient with a neck size greater than 18.5, or a female with a neck size greater than 17.5, has OSA until proven otherwise.
Weight loss, even in moderate amounts, can greatly affect the degree of apnea. A recent study by Tuomilehto et al showed a low calorie diet with lifestyle modifications resulted in significant reductions in patients with moderate OSA. Therefore it is prudent for any overweight patient diagnosed with OSA to initiate a weight loss program as part of the first line of defense against this disease. This may require medical supervision, and proper referral should be made by the surgeon to a clinician with experience in this area. In extreme cases, bariatric surgery may be indicated before any airway surgery.
Continuous Positive Airway Pressure
Continuous positive airway pressure (CPAP) is almost always initiated as part of first-line treatment of OSA and has been the mainstay of therapy for decades. CPAP acts as a pneumatic splint for the airway by pushing air under pressure into the pharynx and preventing collapse of the soft tissues of the airway during sleep. This is accomplished through an air pump and a tight-fitting nasal mask that the patient wears nightly for the duration of their life ( Fig. 113-1 ). In general, only pressurized room air is used, and oxygen is not necessary or indicated.
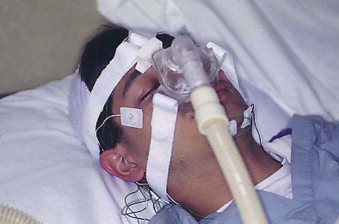
CPAP has been shown to reduce blood pressure, decrease AHI, decrease the results of the Epworth Sleepiness Scale (ESS), improve sleep efficiency, and decrease oxygen desaturation.
When first receiving CPAP, the patient is evaluated by polysomnography while undergoing CPAP titration, increasing the air pressure until respiratory events are maximally diminished. The CPAP trial study can be done on the same night as a shortened-duration standard polysomnography (“split-night study”) or as a separate titration, allowing for longer titration times and the chance for more accurate diagnosis and titration.
Despite the success of CPAP in preventing the symptoms associated with OSA, tolerance of the delivery system is difficult for many people. Patient compliance has been reported to be only 65% to 80%, with 8% to 15% terminating treatment after the first night. Many patients either cannot tolerate the mask or lose it inadvertently during the night. In this situation, other types of delivery systems are available (e.g., nasal prongs and pillows) that may work better for any individual patient, and proper referral to an experienced sleep disorders physician is highly recommended.
The advantages of CPAP are that it provides immediate treatment of the apnea (as opposed to surgery which may take several weeks to relieve apnea), can be totally curative, is titrated to an objective effect, has low morbidity, and is portable. Its disadvantages, other than the common lack of tolerance, are some potential dermatologic effects of the mask, airway drying, and possible barotrauma. For those that can tolerate its use, however, CPAP is still the most common and effective form of phase I treatment for OSA. It is generally when there is failure to tolerate the CPAP, or when patients electively choose to seek surgical options to eliminate the need for CPAP, that surgical treatments are explored.
Continuous Positive Airway Pressure
Continuous positive airway pressure (CPAP) is almost always initiated as part of first-line treatment of OSA and has been the mainstay of therapy for decades. CPAP acts as a pneumatic splint for the airway by pushing air under pressure into the pharynx and preventing collapse of the soft tissues of the airway during sleep. This is accomplished through an air pump and a tight-fitting nasal mask that the patient wears nightly for the duration of their life ( Fig. 113-1 ). In general, only pressurized room air is used, and oxygen is not necessary or indicated.
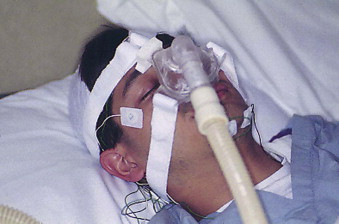
CPAP has been shown to reduce blood pressure, decrease AHI, decrease the results of the Epworth Sleepiness Scale (ESS), improve sleep efficiency, and decrease oxygen desaturation.
When first receiving CPAP, the patient is evaluated by polysomnography while undergoing CPAP titration, increasing the air pressure until respiratory events are maximally diminished. The CPAP trial study can be done on the same night as a shortened-duration standard polysomnography (“split-night study”) or as a separate titration, allowing for longer titration times and the chance for more accurate diagnosis and titration.
Despite the success of CPAP in preventing the symptoms associated with OSA, tolerance of the delivery system is difficult for many people. Patient compliance has been reported to be only 65% to 80%, with 8% to 15% terminating treatment after the first night. Many patients either cannot tolerate the mask or lose it inadvertently during the night. In this situation, other types of delivery systems are available (e.g., nasal prongs and pillows) that may work better for any individual patient, and proper referral to an experienced sleep disorders physician is highly recommended.
The advantages of CPAP are that it provides immediate treatment of the apnea (as opposed to surgery which may take several weeks to relieve apnea), can be totally curative, is titrated to an objective effect, has low morbidity, and is portable. Its disadvantages, other than the common lack of tolerance, are some potential dermatologic effects of the mask, airway drying, and possible barotrauma. For those that can tolerate its use, however, CPAP is still the most common and effective form of phase I treatment for OSA. It is generally when there is failure to tolerate the CPAP, or when patients electively choose to seek surgical options to eliminate the need for CPAP, that surgical treatments are explored.
Positioning Devices
Sleep apnea is often accentuated in the supine position because of gravitational effects on the airway. A simple and conservative approach to reduction of symptoms is sleep positioning. Various devices can be used to force the patient into sleep positions other than supine. A recent study by Skinner et al compared CPAP to the classic “tennis ball technique,” where patients wear a halter top with a tennis ball sewn into the back, preventing them from rolling onto their backs during sleep. This study revealed 13 of 18 patients with mild OSA using the tennis ball technique had a reduction in their AHI to less than 10, which was deemed a success compared to 16 of 18 patients using CPAP. Although not as effective as CPAP, this conservative approach shows some promising results in mild to moderate OSA of positional origin. Other similar devices include special pillows and foam wedges. The avoidance of the supine posture during sleep seems a logical management strategy for position-dependent mild OSA, and several studies have shown the effectiveness of this conservative treatment. The disadvantage to the tennis ball technique is that few patients are willing to use such devices over the long term, and it works only in mild cases where the supine position accounts for the majority of respiratory events.
Oral Appliances
Oral appliances are also considered an acceptable first-line therapy in the treatment of mild to moderate levels of OSA for patients unable to tolerate CPAP or who prefer oral appliance therapy, as indicated in the 2009 Guidelines Publication of the American Academy of Sleep Medicine. Recent data show oral appliances to be effective replacements for patients unable to tolerate CPAP (or who prefer this to CPAP) with mild to moderate sleep apnea.
The basic concept is the same for each of these mandibular repositioning appliances—to prevent retropositioning of the mandible and the hypopharyngeal tongue base when a patient’s musculature relaxes during sleep. In some cases this is accomplished by actually advancing the mandible from its centric occlusion position, whereas others merely maintain the centric occlusion position during sleep (i.e., just preventing retropositioning but not advancing). In addition, they increase the muscular tonus, which may also prevent airway collapse.
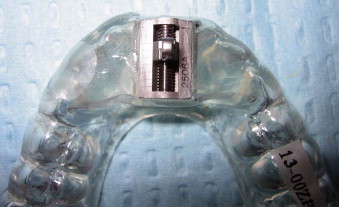
There are currently over 70 appliances used to treat OSA and it is beyond the scope of this chapter to detail each one. They differ in terms of material, range of mandibular movement, amount of dental coverage, rigidity, amount of mandibular advancement, and bite opening. The prime difference is whether the device is fixed, or adjustable and titratable, usually using a jackscrew appliance or class II elastics of varying sizes placed between the arches to control the degree of advancement ( Fig. 113-2 ). Most oral appliances now allow the practitioner to vary the degree of advancement to maximize effect on the apnea while minimizing potential temporomandibular joint (TMJ) and occlusal damage ( Fig. 113-3 ). Some appliances have been designed to attach to the CPAP mask the patient wears at night to facilitate simultaneous use.
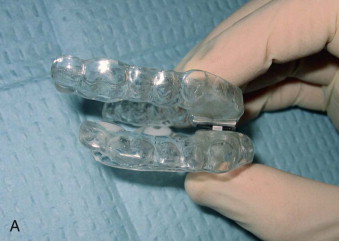
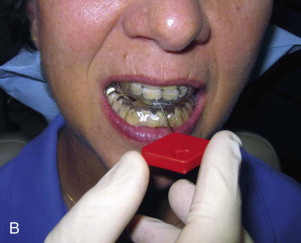
The process of providing a patient with this device involves obtaining an alginate impression of the dental arches, along with an occlusal record, and construction of the splint by a commercial laboratory. Once returned, the device must be inserted and adjusted according to the manufacturer’s recommendation. At that time, it is imperative to insure a proper fit to prevent damage to the teeth, and appropriate bilateral contacts and excursions without interference to prevent myofascial pain symptoms. As the device is worn, intermittent adjustments are required, and the device will likely need to be replaced every year or two.
Once placed, the degree of advancement obtained by the elastics or the jackscrew should be initiated at the centric occlusion level and advanced every few days until the patient’s symptoms disappear or the patient begins to experience significant TMJ discomfort. Maintaining the patient in centric occlusion may be adequate for many patients, because it still prevents the patient’s tongue base from receding during sleep. In some cases, however, it will be necessary to advance beyond the centric occlusion position by 2 to 5 mm to obtain adequate results.
Mandibular advancement devices are currently the most commonly used appliances. However, other devices have been used with varying degrees of success. Tongue-retaining devices that physically hold the tongue in a forward position, as well as palatal lift appliances, have also been used. These appliances only address obstruction at the level of the tongue and soft palate, respectively, and currently have little place in the management of OSA.
Because these appliances can lead to severe TMJ and occlusal disturbances, it is important that oral appliances only be fitted by qualified dental personnel with training and experience in sleep medicine and sleep-related disorders, TMJ disorders, dental occlusion, and associated structures. Even in the best of circumstances, some TMJ tightness may occur in the morning from the chronic advancement, and this may require morning muscle exercises or the use of bite tab stabilizers to assist in replacement of the condyle-disk complex into the fossa. Many courses are now available to general dentists to provide the necessary training. It is imperative, however, that a general dentist work in tandem with a qualified sleep physician and OMFS to provide proper and complete care to the patient.
The success of an oral appliance should allow the patient to feel more alert with fewer arousals, less snoring, and improved breathing. In mild to moderate OSA, the AHI should be reduced by 50% or dropped to a level of ten or fewer events per hour to signal effective use of the device. It is important that placement of the appliance is followed by a repeat PSG to determine effectiveness. This can be done effectively with either a laboratory PSG or with a portable monitoring device. The appliance should also fit comfortably and minimize any dental or skeletal changes through their continued use. Patients often complain of muscle stiffness, TMJ pain, and dry mouth from use of the oral appliances, and unless addressed, these will lead the patient to stop using the device.
These appliances have been shown to be an effective alternative to CPAP, which may be advantageous when noncompliance with CPAP is an issue. In many patients, the use of oral appliances is easier and less intrusive than CPAP, and when applicable, may be substituted.
Palatal Pillars
Patients suffering from snoring with mild to minimal OSA may benefit from the placement of palatal pillars. Pillars are woven polyester tubes approximately 1 inch long that, when placed in the soft palate, can cause scarring and stiffen the tissue to prevent drooping of the palate and subsequent obstruction at this level. Patient selection is of utmost importance in increasing the likelihood this procedure will decrease snoring or manage mild apnea. Obstructions found to be localized in the palate via clinical exam, nasopharyngoscopy and cephalometric analysis, are amenable to this procedure. Patients with a BMI less than 25, small uvulas and flaccid, bulky soft palates are also ideal candidates for this procedure. Importantly, patients should be advised this surgery usually does not completely eliminate snoring, but may change the intensity significantly, allowing the bed partner to remain asleep throughout the night. The devices are also approved by the U.S. Food and Drug Administration for mild sleep apnea management, but most surgeons limit their use to pure snoring or only very mild OSAS at most.
Palatal pillars are easily placed in the office with local anesthesia, and placement takes about 10 minutes. During the procedure, a specially designed, preloaded, injection gun is used to place three implants into the muscular soft palate centered around the midline and 1 cm on each side of the midline ( Fig. 113-4, A and B ). It is imperative that the implants be placed within the muscle so that they do not extrude over time ( Fig. 113-4, C ). Once placed, fibrosis ensues, thereby stiffening and adding support to the soft palate. This technique has been shown to be moderately effective in reducing snoring and daytime somnolence.
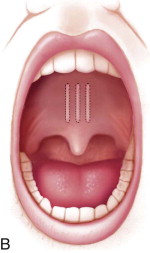
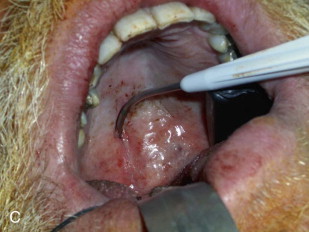
Following the procedure, patients are placed on a diet of soft, cold foods for 24 hours, after which they are able to return to a general diet. Patients may complain of temporary fullness at the back of the throat and some mild soreness for 1 to 2 days but should be assured that this will be very short-lived. Most patients are relatively asymptomatic and return to work the next morning. Close follow-up is essential to monitor rejection of the implant or extrusion through the mucosa within the first few weeks postoperatively. The stiffening process may take up to 90 days for maximum effect to be noticed.
Radiofrequency Ablation
Radiofrequency devices use radiofrequency energy to cauterize and create a series of known and controlled submucosal volumetric lesions, which lead to thermal contraction and ablation within tissue. This method has been used for more than a century, and more specifically for the treatment of OSA, for the past decade with varying degrees of success in the tongue, nasal turbinates, and in the soft palate. Initially, surgical electrocautery and lasers were used within the tissue; however, because of the charring and lack of control over tissue effects, there was significant scarring and excessive contraction. Studies have shown that a lower temperature, with longer duration and repeated procedures, has a higher success rate with minimal tissue necrosis.
In this procedure, temperatures from 120° F to 200° F are delivered to the tissues via a handpiece electrode. The sheathed electrode allows for the tip to work deep within the tissue, preventing superficial loss of tissue. The surrounding tissues undergo vacuolar degeneration with subsequent internal scarring. This scarring causes the tissue to stiffen, and its size and flaccidity are diminished.
A small to moderate amount of local anesthesia (approximately 2 mL) with a vasoconstrictor is delivered to the soft palate by injection after the area has been topically anesthetized with benzocaine, butamben/tetracaine/benzocaine (Cetacaine), or lidocaine. The electrode is placed into the soft palate with the tip of the electrode positioned just superior to the uvula and inserted to a depth such that the sheathed part of the electrode protects the superficial mucosa. The exposed electrode is inserted solidly into the submucosal tissues, where the tip reaches a temperature of 120° F within approximately 10 seconds. A single lesion in the palate is created and the electrode is then removed from the tissue. In the tongue, a midline lesion is created and two additional sites are placed lateral to the initial midline lesion to complete the ablation.
Postprocedure, patients may feel a sense of fullness at the back of their throats and complain of a sore throat. Nonopioid analgesics are generally sufficient for any postoperative pain. The healing process may take 3 to 4 months with the need for additional procedures before the patient notices stiffening and volume reduction. Patients often tend to notice a diminished intensity of snoring after the first procedure, however. A second procedure is typically necessary if snoring continues 6 months after the initial ablation. Although many of these radiofrequency devices were sold, the procedure is not used as often as when it was introduced because of its lack of predictability.
Uvulopalatopharyngoplasty
Uvulopalatopharyngoplasty (UPPP) has been historically, along with nasal septoplasty, the most commonly used procedure to treat OSA. This was primarily because it was the only viable surgical procedure available until relatively recently, when the surgical options for OSA treatment expanded. In fact, this procedure was never truly a very effective surgery unless the primary site of obstruction was the soft palate. Since we now know that the majority of obstructions occur at multiple levels within the airway, targeted procedures such as UPPP are now generally considered only a single component of a more complex phase I treatment plan and are rarely done alone.
As with any targeted procedure, thorough examination, imaging, and nasopharygoscopy should be performed to determine if the likely source of obstruction is in the soft palate. The nasopharyngoscopy aids the surgeon not only in determining the level or levels of obstruction, but also in ruling out any neoplastic or pathologic processes, which though unlikely, may be present and causing the obstruction. Only when the level or levels of obstruction are ascertained can the surgeon determine if UPPP will be a viable treatment option (either as the sole procedure or in combination with other procedures) for the patient with OSA.
UPPP has traditionally been performed in the hospital operating room under general anesthesia and has been reported to have varying degrees of complications ranging from velopharyngeal insufficiency, dysphagia (difficulty swallowing), voice changes, and death from general anesthesia.
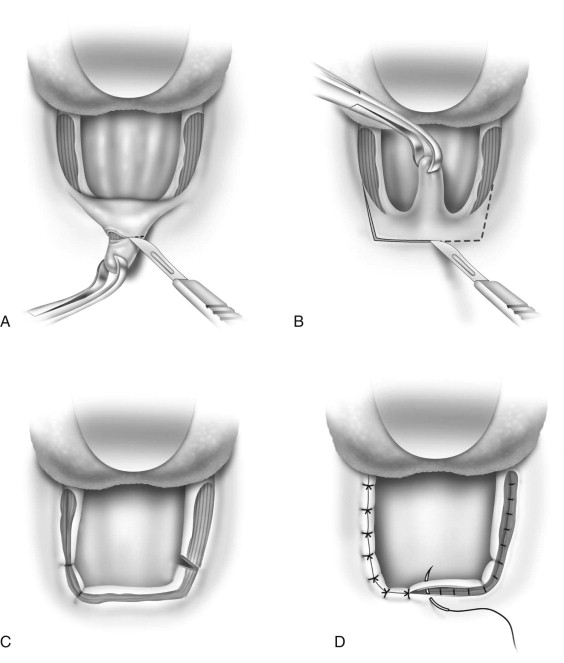
First developed by Ikematsu, who followed habitual snorers for several years, the procedure entailed performing an adenoidectomy, tonsillectomy, and uvulectomy and excising the redundant lateral pharyngeal wall mucosa. The original technique has been modified to varying degrees, but the goal of shortening the palate and thereby widening the airway has remained consistent.
If the tonsils are to be removed a tonsillectomy may be performed by any standard technique. The uvula is then grasped with an Allis forceps and everted to expose the posterior aspect. A curvilinear incision is made at the base of the uvula extending to each tonsillar pillar bilaterally. A trapezoid-shaped incision is outlined in the mucosa of the anterior soft palate that extends above the uvula ( Fig. 113-5 ). The uvula is then excised, which then allows the posterior soft palate to be closed to the anterior soft palate superiorly. The posterior and anterior tonsillar pillars bilaterally are closed primarily ( Fig. 113-6 ).
Laser-Assisted Uvulopalatoplasty
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


