Introduction
The aim of this study was to compare the nickel released from 3 kinds of orthodontic brackets: new conventional stainless steel, recycled stainless steel, and nickel-free brackets.
Methods
This in-vitro study was performed by using a classic batch procedure. Samples were immersed in artificial saliva at various acidities (pH 4.2, 6.5, 7.6) over an extended time interval (0.25, 1, 24, 48, and 120 hours). The amount of nickel released was determined by using an atomic absorption spectrophotometer and an inductively coupled plasma atomic emission spectrometer. Statistical analysis included a linear regression model for repeated measures, with calculation of Huber White robust standard errors to account for intrabracket correlation of data. For post-hoc comparisons, the Bonferroni correction was applied.
Results
The recycled brackets released the most nickel (74.02 ± 170.29 μg per gram); the new stainless steel brackets released 7.14 ± 20.83 μg per gram. The nickel-free brackets released the least nickel (0.03 ± 0.06 μg per gram). All the differences among the groups were statistically significant ( P = 0.000).
Conclusions
Reconditioned brackets released the most nickel. Moreover, the highest nickel release was recorded in the 2 experiments performed at pH 4.2; it was lower at pH 6.5 and 7.6. Conversely, no relevant differences were observed overall between the maxillary and mandibular arches.
In orthodontics, nickel is a commonly used metal because it is a component of superelastic shape-memory wires, as well as stainless steel and other alloys in different percentages. Release of nickel from metallic orthodontic appliances has been observed in several in vitro studies, whereas ionic release in vivo in the oral cavity is more difficult to demonstrate, although corrosion has clearly been evident in orthodontic appliances after treatment. The amount of nickel released from fixed orthodontic appliances in vitro varies depending on the manipulation of the appliances and different physical and chemical test conditions. Park and Shearer reported an average release of 40 μg of nickel per day from a simulated full-mouth fixed appliance. The release of nickel was not necessarily proportional to the alloy’s nickel content.
Factors such as temperature, quantity and quality of saliva, salivary pH, plaque, amount of protein in saliva, physical and chemical properties of food and liquids, and general and oral health conditions can influence corrosion in the oral cavity.
Previous reports have documented increased amounts of nickel released from recycled orthodontic appliances. Ionic leaching can be accelerated by heat treatment during the reconditioning process: it has been demonstrated that the release of metals from stainless steel appliances increases after heating to 400°C to 800°C.
There is great concern about the potential biologic hazards from alloys that contain nickel and chromium. Nickel is known to be a strong immunologic sensitizer and the most common cause of allergic contact dermatitis, which is a type IV delayed hypersensitivity immune response. It is estimated that 4.5% to 28.5% of the population have hypersensitivity to this metal, with higher prevalence in females. However, there have been a few reports of contact stomatitis from nickel in orthodontic patients. Moreover, some authors pointed out the role of nickel accumulation and epithelial cell proliferation in orthodontic treatment-induced gingival overgrowth. Dermatoses at sites other than the mouth—eg, flares at sites of previous nickel dermatitis or hand eczema—have been seen as sequelae to orthodontic treatment. Finally, increased urinary nickel levels 2 months after the placement of orthodontic appliances have been reported.
In-vitro experiments on cultured human gingival fibroblasts showed that ions released from implanted nickel-chromium alloys caused altered cellular functions. In a recent study, Faccioni et al found that nickel ions can produce DNA breaks in cells of the oral mucosa. Other metals, such as chromium, can also cause hypersensitivity, dermatitis, and asthma. This metal can induce other adverse biologic effects, such as cytotoxicity, and it is a suspected genotoxic.
Now, the use of nickel-free brackets is preferred because those orthodontic appliances should not have any allergic or toxic effect, but, to our knowledge, no studies have attempted to measure the metal released from nickel-free brackets.
Accordingly, the purposes of this investigation were to evaluate and compare the amounts of nickel released from 3 kinds of metallic orthodontic brackets: new conventional stainless steel brackets, recycled stainless steel brackets, and nickel-free brackets. This in-vitro study was performed with a classic batch procedure by immersion of the samples in artificial saliva at various acidity levels over an extended time interval.
Material and methods
All laboratory ware, made of low density polyethylene (Essedi Plastik, Senago, Italy), was cleaned by soaking in 1:1 nitric acid for at least 48 hours, rinsed with double-distilled water, air dried in a hood, and stored in plastic bags. All reagents were of analytical or pure grade to avoid contamination. Solutions were prepared with ultrapure water (Milli-Q, Millipore, Billerica, Mass). Standard solutions of nickel were used to prepare daily diluted solutions for the instruments’ calibrations.
A research pH meter (PHM 84, Radiometer, Copenhagen, Denmark) and a combined ORION glass electrode (Thermo Electron, Waltham, Mass) were used for the pH measurements.
A graphite furnace atomic absorption spectrophotometer (AA-6601G/GFA 6500, Shimadzu, Kyoto, Japan) was used for nickel determination when its concentration in the samples was less than 10 mg per liter. Instrumental parameters and thermal programs were the same as previously reported : wavelength (nm), 232.0; band width (nm), 0.5; background correction, D2 lamp; thermal program, drying at 150°C to 250°C for 30 seconds, ashing at 1200°C for 13 seconds, and atomization at 2500°C for 3 seconds.
An inductively coupled plasma atomic emission spectrometer (ICP JY2301, Horiba Jobin Yvon, Longjumeau, France) was used for nickel determination when the metal concentration in the samples was higher than 10 μg per liter.
A total of 1080 brackets, divided into 3 groups, were evaluated: (1) 360 new conventional Victory stainless steel brackets (slot, 0.022 in, MBT prescription, 3M Unitek, Monrovia, Calif); composition (% weight): chromium, 10% to 20%; nickel, <4%; (2) 360 Victory stainless steel brackets (slot, 0.022 in, MBT prescription, 3M Unitek), recycled by Alpident (Villarperosa (TO), Italy); the recycling process involved washing the brackets in a nonacid solution, followed by drying and heating to 350°C for 24 hours; the brackets were then washed twice in a nonacid solution, dried, electro-polished for 20 seconds, and sterilized at 250°C ; (3) and 360 Sprint nickel-free brackets (slot, 0.022 in, MBT prescription, Forestadent, Pforzheim, Germany); composition (% weight): chromium, 16% to 20%; nickel, <0.3%.
The artificial saliva, used as the immersion test electrolyte, was modified according to the method of Tani and Zucchi with the following chemical composition: 5.3 10 −3 mol/L KSCN, 1.5 10 −2 mol/L NaHCO 3 , 2 10 −2 mol/L KC1, 1.4 10 −3 mol/L NaH 2 PO 4 , 0.1 g/L CH 4 N 2 O (urea), and 0.1 mg/L α-amylase from human saliva. This solution was divided into 3 aliquots; in each, the pH was measured potentiometrically and adjusted with a small amount of hydrochloric acid or base (sodium hydroxide) so that the volume of the solution remained constant. The final pH values of the 3 aliquots were 4.2, 6.5, and 7.6.
To simulate the release of nickel from a mouth quadrant (from central incisor to second premolar), 5 brackets were considered a specimen (quadrant). For each of the 3 pH values of the artificial saliva (4.2, 6.5, 7.6), 12 maxillary and 12 mandibular quadrants (each consisting of 5 brackets) were measured, according to the protocol in Table I . Thus, each of the 3 bracket groups (n = 360) was subdivided into 6 subgroups (n = 60), depending on the pH of the artificial saliva and the mouth quadrant.
| Brackets | pH | Maxillary quadrant | Mandibular quadrant |
|---|---|---|---|
| Victory (3M Unitek) n = 360 | 4.2 | n = 12 (5 brackets each) | n = 12 (5 brackets each) |
| 6.5 | n = 12 (5 brackets each) | n = 12 (5 brackets each) | |
| 7.6 | n = 12 (5 brackets each) | n = 12 (5 brackets each) | |
| Victory reconditioned (3M Unitek + Alpident) n = 360 | 4.2 | n = 12 (5 brackets each) | n = 12 (5 brackets each) |
| 6.5 | n = 12 (5 brackets each) | n = 12 (5 brackets each) | |
| 7.6 | n = 12 (5 brackets each) | n = 12 (5 brackets each) | |
| Sprint (Forestadent) n = 360 | 4.2 | n = 12 (5 brackets each) | n = 12 (5 brackets each) |
| 6.5 | n = 12 (5 brackets each) | n = 12 (5 brackets each) | |
| 7.6 | n = 12 (5 brackets each) | n = 12 (5 brackets each) |
The specimens were rinsed with a mixture of 1:1 ethanol and acetone in an ultrasonic cleaning bath for 30 minutes and then air dried under a cleaned hood. Each group of dried brackets was weighed (analytical balance RE 1614, Sauter, Ebingen, Germany) to facilitate an estimate of the metallic mass exposed to the solutions. Then the samples were immersed in cleaned, low density polyethylene in 100-mL bottles containing 10 mL of artificial saliva; during the immersion period, they were gently stirred on a shaking plate at room temperature. This procedure ensured that all parts of the brackets were soaked, thus obtaining homogeneous solutions. A blank sample was prepared for each experiment with 10 mL of artificial saliva in a cleaned 100-mL bottle.
After 15 minutes, 1 hour, and 24, 48, and 120 hours, 0.1 mL of the eluent was removed, diluted, and acidified at pH 2 with HNO 3 . Each solution was analyzed to determine the concentrations of nickel released from the brackets, by using the graphite furnace atomic absorption spectrophotometer or the inductively coupled plasma atomic emission spectrometer. Nickel concentration was expressed in micrograms of metal released per gram of the brackets.
The pH of the eluent solutions was always remeasured after each cycle, and it was always found equal to the original value within the experimental error (± 0.1 pH unit).
At the end, the brackets were rinsed with ultrapure water (Milli-Q), air dried under a cleaned hood, and reweighed to verify whether any material was lost during the leaching procedure.
Statistical analysis
With their skewed distribution, the data were described as medians and interquartile ranges (IQR). The area under the curve (AUC) of nickel levels over time and the maximum value were estimated and summarized as medians and 95% confidence intervals.
The primary analysis aimed at comparing nickel released over time from the 3 brackets (while adjusting for the different experimental settings). A multiple general linear model was used: it included the main effects for bracket and time, as well as their interaction, and terms for pH and quadrant. Huber-White robust standard errors were calculated to account for intrabracket correlation of data, time, and experimental setting. The comparison of time profiles for the nickel release between brackets was assessed by testing for interaction. For the analysis, nickel was log-transformed. Model adequacy was verified by inspection of the residuals. Changes over time in the bracket groups were assessed in separate models. To reflect the experimental design, a secondary analysis was performed: the same regression models were fitted for the 6 combinations of pH (4.2, 6.5, 7.6) and quadrants (mandibular and maxillary arches).
Computations were performed with Stata software (version 10, Stata, College Station, Tex) and Medcalc (version 9.5.1.0, Frank Schoonjans, Mariakerke, Belgium). All tests were 2-sided. P <0.05 was considered statistically significant for the main analysis, whereas a more conservative P <0.001 was retained for the post-hoc comparisons and subgroup analyses.
Results
As shown in Table II , the median nickel release over time was highly dependent on the type of brackets tested ( P <0.001 for interaction, while adjusting for baseline nickel concentration, pH, and quadrant at the multivariable analysis). It was lowest for the new stainless steel Sprint brackets, slightly higher for the Victory brackets (vs Sprint, P <0.001) and highest for the Victory reconditioned brackets (vs Sprint and Victory, both P <0.001). The overall nickel release, measured by the AUC, ranged from 3.10 (Sprint) to 1800 μg per hour per gram (Victory reconditioned), and the peak concentrations were from 0.08 (Sprint) to 22.24 μg per gram (Victory reconditioned). For all brackets, we observed significant changes in nickel concentrations over time ( P <0.001) ( Table II , Fig 1 ).
| Bracket | Median nickel concentration ∗ (IQR) | AUC of nickel concentration over time median (95% CI) | Peak nickel concentration median (95% CI) | Comparison of brackets over time ( P value for interaction) † | Changes over time in bracket groups ( P value) † |
|---|---|---|---|---|---|
| Overall | <0.001 | ||||
| Sprint | 0.00 (0.00-0.02) | 3.10 (2.36-4.26) | 0.08 (0.04-0.12) | Sprint vs Victory <0.001 | <0.001 |
| Victory | 0.34 (0.10-3.08) | 139 (55-342) | 2.37 (0.70-6.06) | Victory vs VictoryR <0.001 | <0.001 |
| VictoryR | 12.27 (0.83-35.0) | 1800 (1424-2542) | 22.24 (19.47-36.13) | VictoryR vs Sprint <0.001 | <0.001 |
∗ Collapsed over time and experimental setting.
† Computed from a multivariable general linear model, while adjusting for baseline nickel concentration and experimental setting (pH and quadrant); model P <0.001; R 2 = 0.76; VictoryR , Victory reconditioned brackets.
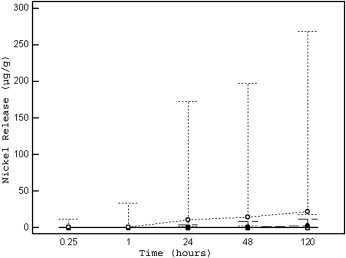
Table III and Figure 2 summarize the results for the 6 experimental settings, according to the combination of pH and quadrant. Overall, the highest nickel release was recorded in the experiments performed at pH 4.2 (median release, 1.13; IQR, 0.00-44); it was lower at pH 6.5 (median, 0.23; IQR, 0.03-1.83) and pH 7.6 (median, 0.15; IQR, 0.01-1.07) ( P <0.001, while adjusting for baseline nickel concentration, brackets, and quadrant in the multivariable analysis). Conversely, no relevant differences were observed overall between the maxillary and mandibular arches (median, 0.16; IQR, 0.01-1.32, vs median, 0.36; IQR, 0.02-11; P = 0.34, while adjusting for baseline nickel concentration, pH, and brackets at the multivariable analysis). When assessing separately each experimental setting, the results were similar to those of the general analysis: the lowest median nickel release over time, total release (from AUC), and median peak release were observed for the Sprint brackets, with a slightly higher value for the Victory and the highest value for the Victory reconditioned brackets ( P <0.001). Similarly, highly significant pairwise differences between the 3 times were recorded ( P <0.001). Finally, significant changes in nickel release over time were recorded ( P <0.001) in all situations, except for the Sprint brackets studied at pH 7.6.



