Fig. 6.1
(a) The DIAGNOdent pen (KaVo) can be inserted in the approximal space to detect caries below the contact area. It should be inserted from both the buccal and the lingual aspect. (b) The sapphire tip of the DIAGNOdent pen directs the laser light toward the tooth surface and captures the fluorescence signal
More recently, camera-based devices (VistaProof and VistaCam iX, both Dürr, Germany) and system were marketed and tested in vitro (Jablonski-Momeni et al. 2011) and in vivo (Diniz et al. 2012; Jablonski-Momeni et al. 2013, 2014). The clinical diagnostic accuracy was reported to be 0.46 for enamel lesions and 0.91 for dentine lesions. However, the reported sensitivity value at the dentine threshold was 0.26 (specificity being 0.98), while at the enamel threshold sensitivity was found to be 0.92 (specificity 0.41). It was concluded that the device could support the treatment decision in combination with meticulous visual-tactile inspection (Jablonski-Momeni et al. 2014). The advantage of such a camera-based system however is the possibility to quantify the caries process and to store the captured images in order to monitor lesions and allow a comparison at later recall appointments. Thus, invasive treatment could be postponed and only be performed when a lesion clearly shows to be progressive.
6.3 Quantitative Light-Induced Fluorescence (QLF)
Excitation of dentine with blue light (370 nm) causes it to fluoresce in the green-yellow spectrum. The method that uses this particular wavelength is known as quantitative light-induced fluorescence. The fluorescence is observed through a yellow filter (λ ≥ 540 nm) to cut out the excitation light. An incipient enamel lesion can be observed because of an increase of light scattering relative to the surrounding enamel. Two effects thus occur: 1) because less excitation light reaches the dentine, less fluorescence is produced underneath the lesion; and 2) less fluorescent light is observed because it is scattered through the lesion. Consequently, the contrast between the surrounding sound enamel and the lesion is enhanced. An incipient lesion can be seen as a dark spot on a light green background. In addition to green fluorescence from dentine, the fluorescence of bacterial porphyrins is visible in red (Neuhaus et al. 2009).
The QLF method was originally developed for intraoral quantification of mineral loss in enamel lesions. A color microvideo CCD camera and computed image analysis were assembled and used (Inspektor Research Systems, the Netherlands) (de Josselin de Jong et al. 1995). The software subtracts the digital fluorescence images of the enamel. Three lesion quantities are thus being calculated: fluorescence loss (mean ΔF or ΔFmax), area of the lesion (in mm2), and their product (ΔQ).
In order to enhance the application in clinical studies at different locations, a smaller, portable system for intraoral use was developed. Data is collected, stored, and analyzed by custom-made software. The portable QLF device was validated against chemical analysis and microradiography for the assessment of mineral changes in enamel and compared with results from laser-light measurements (Pitts and Longbottom 1987). It was concluded that QLF was a valid method for quantification of incipient enamel lesions. However, accurate assessments were limited to a depth of about 400 μm. Thus, the QLF method could be regarded as sensitive enough to measure remineralization in early enamel lesions. Moreover, attempts to establish suitable cutoffs for dentin lesions were made (Longbottom and Pitts 1990).
The in vivo reliability of QLF is excellent for the quantification of smooth-surface caries, with intraclass correlation coefficients for interexaminer reproducibility of r = 0.95–0.99 (Lagerweij et al. 1999; Tranaeus et al. 2002). The QLF method has been applied in a number of clinical trials. The QLF method has been applied to test the natural behavior of white spot lesions after removal of orthodontic brackets (van der Veen et al. 2007; Mattousch et al. 2007), for the evaluation of preventative measures in patients at high caries risk (Al-Khateeb et al. 1998), and for comparing different prophylactic means in clinical studies (Tranaeus et al. 2001; Karlsson et al. 2007). In a clinical trial with 34 fifteen-year-old students with non-cavitated occlusal surfaces, QLF was more sensitive than meticulous visual inspection and yielded double the number of carious sites (Kuhnisch et al. 2007). Recently, the QLF method was used as a gold standard in 39 children at nursery schools to compare the effect of a remineralizing agent (CPP-ACP) with toothbrushing with a fluoridated toothpaste (Sitthisettapong et al. 2015). Although QLF is a sensitive and accurate method to assess and monitor enamel lesions, its time-consuming image processing and analysis and its costs are the biggest obstacles for wide use in private dental practice.
An intraoral QLF camera system was lately marketed that offers the choice between white light mode and fluorescent light mode (Soprolife, Acteon, France). The fluorescence images are not quantitative but allow qualitative discrimination between autofluorescence and bacterial fluorescence. Effort to translate the images into a clinically relevant classification system has recently been made (Rechmann et al. 2012). It was shown that the Soprolife camera with the blue fluorescence mode yielded a sensitivity at dentine threshold of 0.95 and a specificity of 0.55 in vitro (Rechmann et al. 2012). It must be kept in mind that such a low specificity means a high fraction of false-positive findings, i.e., many teeth would have received unnecessary invasive treatments. Consequently, such a camera-based system should never be a stand-alone device for decision-making. Despite of the limited clinical value in caries diagnostics, with regard to periodontal diseases the same device was shown to provide reliable information about the presence of microbial plaque and gingival inflammation (Rechmann et al. 2016) (Fig. 6.2).
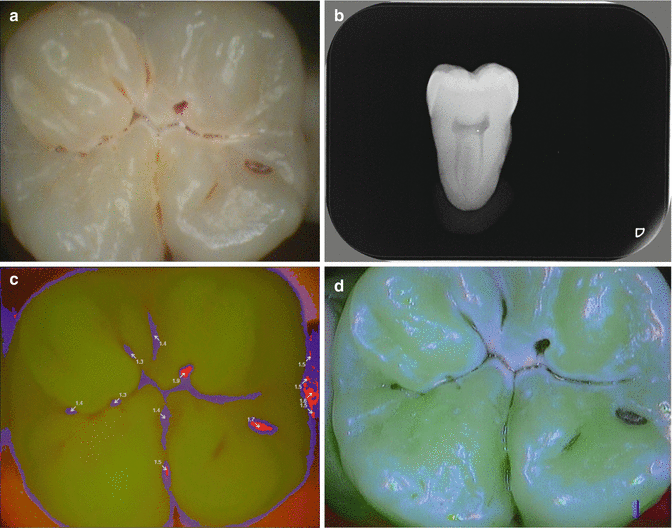

Fig. 6.2
(a) Occlusal surface, clinical view. (b) Radiograph shows initial dentine caries. (c) VistaProof camera (Dürr) indicates three spots that need some kind of invasive treatment (red areas). (d) Soprolife camera (Acteon) indicates a carious process in the main fissure (light red areas)
6.4 Fiber-Optic Transillumination (FOTI)
The method of tooth transillumination with an appropriate intense light source is widely accepted by dental practitioners for caries detection in anterior teeth. For this purpose, FOTI is simple and quickly to apply, and dentists usually have polymerization lamps at hand which might also be used.
Fibre-optic transillumination (FOTI) uses the principle of light scattering to increase contrast between sound and carious enamel. The light source is applied at an accessible smooth surface of a tooth. Light transmission is then observed from either the opposing side in front teeth or from the occlusal aspect in premolars or molars. Carious enamel appears dark in FOTI, because it is demineralized and porous, and light is scattered more than in healthy and sound enamel. Dentine caries appears as an orange or brown shadow from underneath the enamel. This observation helps to discriminate between lesions that are restricted to enamel and those reaching into dentin. More recently high-intensity LED light sources have become available that are inexpensive and have the potential to be widespread in dental offices. Most of the early FOTI research concentrated on the detection of proximal lesions and the performance with respect to this has been reviewed by (Vaarkamp et al. 2000). It was concluded that the specificity of both FOTI and bitewing radiography was high but that the sensitivity of FOTI was significantly lower than bitewing radiography. FOTI could therefore be used as a complementary method in the caries diagnostic process. In digital FOTI (DIFOTI), a CCD sensor replaces the human eye (Schneiderman et al. 1997; Young and Featherstone 2005). It was reported that compared to bitewing radiography DIFOTI performs significantly better in enamel lesions, but specificity in dentine lesions was significantly lower (Astvaldsdottir et al. 2012). Intra- and interobserver agreement were reported to be similar between DIFOTI and both digital and film radiography (Astvaldsdottir et al. 2012). It should be kept in mind that up to now no clinical trial has been conducted using DIFOTI as a sole diagnostic method. Regarding other applications of FOTI, it should me mentioned that especially for detecting enamel cracks FOTI is a very helpful method (Ellis 2001). (DI)FOTI may support instantaneous decision-making in the dental office, but for monitoring lesions or assessing the effect of preventative measures, it is not suitable.
6.5 Near-Infrared Transillumination
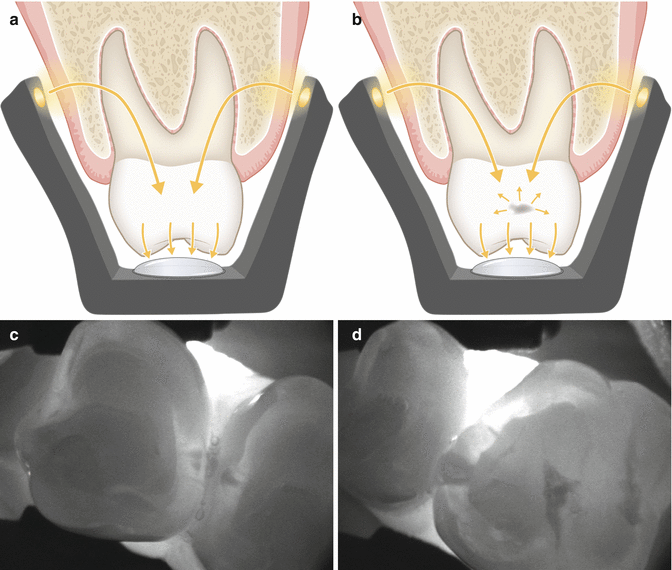
A new camera-based device (DIAGNOcam, KaVo, Biberach, Germany) uses near-infrared light transillumination (NILT) for caries detection in posterior teeth. This method can be understood as further development of the DIFOTI and uses invisible long-wave light (λ ~780 nm) instead of visible light. Another modification is that the illuminating light enters the body at the alveolar process, far below the interproximal space. Both modifications improved considerably the quality of diagnostic imaging of interproximal sites from the occlusal aspect (Fig. 6.3a, b). The device has most recently been marketed, and an ongoing clinical validation study showed that NILT-based treatment decisions to excavate caries were in most cases the same as those by conventional bitewing radiographs (Kühnisch et al. 2015). Caries depth into dentine cannot be reliably visualized with NILT. Dentine affection is displayed only sporadically, but deep dentine lesions become regularly visible in NILT (Kühnisch et al. 2015). Therefore, the shape of the shadow in enamel has to be taken into account when assessing lesion depth (Söchtig et al. 2014). Only shadows that touch the enamel-dentine border with a broad base can be regarded as established dentine lesions. With respect to early caries lesions restricted to the enamel, there seems to be a learning curve with this technology, because the clinical performance of NILT was reported to be moderate for enamel lesions and not better than bitewing radiography (Jost et al. 2015). Next to handling issues, the projection of anatomic surface features such as steep slopes or grooves could be misinterpreted as enamel caries.
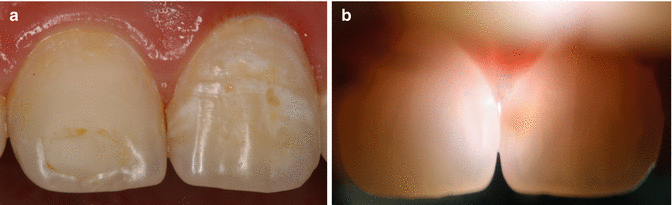

Fig. 6.3
FOTI can be easily applied on front teeth (a) and displays caries nicely (b)
The NILT technology is not capable to detect proximal cavities, nor can it display secondary caries at cervical restoration margins. Additionally, in children the visibility of the crowns might be severely hampered by physiological root resorption, which disrupts the path of incoming light. Furthermore, enamel lesions that are infiltrated or treated with self assembling peptides are visible to the same degree after the treatment as before. The NILT method could, however, be used for screening purposes. In questionable patients further diagnostic measures such as radiography can then be applied in a more tailored way. Thus, especially younger patients could be prevented from too often applied ionizing radiation (Fig. 6.4).