Fig. 1.1
Periodic table showing the elements in the major groups and transition metals. Only the elements associated with life are listed. The common elements are in dark green. Light orange/yellow or pink/white backgrounds indicate the metal and halide elements that occur as ions. Trace elements are indicated in red, pink, or light green (Modified from the full Periodic Table found in Wikipedia at http://en.wikipedia.org/wiki/Periodic_table)
The nucleus of the first element, hydrogen, consists only of a proton. The second, helium, has two protons and two neutrons. The third, lithium, has three protons and three neutrons, and so on (Fig. 1.2). The atomic weight, measured as daltons (Da), is the sum of the number of protons and neutrons in an element’s atomic nucleus. Hydrogen has a mass of 1 Da (the mass of a proton). Helium has two protons and two neutrons to hold the protons together. It therefore has a mass of 4 Da. Lithium has three protons and three neutrons and therefore a mass of 6 Da (Fig. 1.3). Oxygen has an atomic number of 8 and atomic mass of 16.

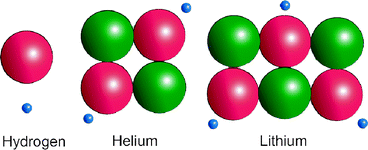

Fig. 1.2
Proton, neutron and electron

Fig. 1.3
Atomic composition of hydrogen, helium, and lithium, the first three elements of the periodic table. (Diagram prepared by Dr Wirsig-Weichmann)
Isotopes are elements possessing different numbers of neutrons. Deuterium, a hydrogen atom containing a neutron in addition to a proton, has the same chemical properties as hydrogen but twice the atomic mass (heavy hydrogen), 2 Da instead of 1 (Fig. 1.4). There is also a rarely occurring third isotope of hydrogen in nature, tritium. Tritium is important because it is radioactive; one of its neutrons is unstable and releases a beta (β) particle. Beta particles resemble fast-moving, free electrons that may be positively charged (positrons), or more commonly negatively charged (electrons). The release of a β-particle converts one of the two neutrons of tritium (Fig. 1.3) to a proton, making a stable isotope of helium (two protons and one neutron, Fig. 1.4). Table 1.1 lists the radioactive elements important in biology, the nature of the emitted radioactivity, what element they decay to, and their usual use in biology.
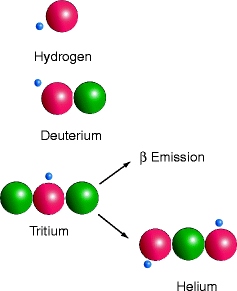

Fig. 1.4
Isotopes of hydrogen. Tritium is unstable. It changes to helium by a neutron emitting a β-particle and becoming a second proton. The β-particle is a radioactive emission. (Diagram prepared by Dr Wirsig-Weichmann)
Table 1.1
Radioactive isotopes important in biology
|
Element
|
Symbol
|
Isotope
|
Symbol
|
Decays to
|
Symbol
|
Primary Use
|
|---|---|---|---|---|---|---|
|
Hydrogen
|
1H
|
aTritium
|
3H
|
Heliumb
|
3He
|
Metabolic pathways
|
|
Carbon
|
12C
|
aCarbon-14
|
14C
|
Nitrogen
|
14N
|
Metabolic pathways
|
|
Phosphorus
|
31P
|
cPhosphorus-32
|
32P
|
Sulfur
|
32S
|
Phosphorylations
|
|
Calcium
|
40Ca
|
aCalcium-41
|
41C
|
Potassium
|
41K
|
Bone metabolism
|
|
Iodine
|
127I
|
aIodine-131
|
131I
|
Xenon
|
131Xe
|
Thyroid cancer therapy
|
|
Iodine
|
127I
|
dIodine-125
|
125I
|
Telurium-125d
|
125Te
|
Protein labeling
|
By the third decade of the twentieth century, the emitted radioactivity from artificially synthesized tritium (3H) and carbon-14 (14C) was used to follow biochemical reactions. Glucose, amino acids, and other chemical compounds were synthesized with 3H or 14C incorporated either randomly or at some known position in the molecule. The radioactive products derived from radiolabeled glucose or amino acids added to cells, tissue slices, or a whole organism identified the metabolic fate of these molecules under defined conditions. These studies led to the establishment of the metabolic pathways that we now take for granted such as protein, fatty acid, amino acid, and nucleic acid metabolism.
1.2 Isotopes Date Paleontology Samples Such as Teeth
Another use of radioactive carbon-14 is to date prehistoric bones and teeth, the longest-surviving parts of the body. Carbon-14 (14C; six protons and eight neutrons) is continually formed in the upper atmosphere by cosmic rays acting on nitrogen (14N; seven protons and seven neutrons). The energy of the cosmic rays causes a proton and electron in atoms of 14N to fuse into a neutron. The newly formed 14C is rapidly oxidized to 14CO2, which enters the earth’s plant and animal life through photosynthesis and the food chain. The ratio of 14C to nonradioactive carbon is approximately constant over time. When plants or animals die, carbon uptake ceases, 14C is not replenished, and it slowly decays with a half-life of close to 5,700 years. The radioactivity of a sample whose age is not known can be used to indicate the amount of 14C remaining, provided it is not more than 40,000 years of age. After that time, so much 14C has decayed that what is left is not measurable. From the ratio of radioactive carbon to total carbon content, it is possible to determine how much was lost relative to the current ratio (unchanged over billions of years) and therefore how long ago the bone or tooth was part of a living organism.
1.3 Isotopes Indicate Ancient Life Forms and Climate Changes
Not all isotopes are radioactive, and even some stable isotopes can be useful. A stable isotope of carbon, carbon-13 (13C; 6 protons and 7 neutrons) has accounts for about 1% of carbon atoms on earth. Because it is chemically more stable than 12C, living organisms preferentially utilize 12C for chemical reactions (metabolism). Therefore, rocks containing a greater than usual 12C/13C ratio are potentially ‘chemical fingerprints’ of life. Minute residues in some of the oldest rocks on Earth, from Akilia Island near Greenland, have a chemical fingerprint that may have come from living organisms. Analysis of selected tiny samples using an ion microprobe revealed ratios of carbon-13 to carbon-12 that were 2–5% less than expected. Some prebiotic process may have enriched the rock with carbon-12 atoms, and it lay apparently undisturbed (based on the surrounding crystal structure) for over 3,700 million years (3.7 Giga-years, Gyr).
The age of ancient rock samples is independently determined from the rate of decay of another isotope, 87Rubidium (87Rb) to 87Strontium (87Sr). The half-life of this decay is about 1.0 Gyr. Amounts of 87Sr and 87Rb are measured, and a ratio is derived and compared with the ratio of 87Sr to stable strontium (86Sr) in the rock sample. The age of the rock sample is then derived mathematically from these ratios, given the half-life of 87Rb.
The common element of oxygen has eight protons and eight neutrons. A stable isotope with two additional neutrons is also relatively common. Because water-containing 18O evaporates more slowly and condenses more rapidly, the ratio of 18O/16O in Antarctic ice cores indicates major climate shifts since early in geologic time. Increased 18O indicates a sudden warming, and increased 16O indicates a sudden cooling.
1.4 The Elements in Biology
The electrons of the elements are arranged in shells surrounding the nucleus. The first shell consists of two electrons, the second and third each of eight electrons, and the fourth and fifth each of 18 electrons. Elements take part in a chemical reaction by gaining or sharing electrons to complete their outer shell except for the noble gases (helium, neon, argon, krypton and xenon, far right-hand column of the periodic table). These elements already have a complete outermost electron shell. They therefore have no chemical reactivity and are incompatible with life.
Figure 1.1 shows the elements important for all of life. The common elements are depicted in dark green boxes: carbon, hydrogen, nitrogen, oxygen, phosphorous, and sulfur. Elements depicted in light orange/yellow or pink/white boxes make up the major cations and anions of living organisms: sodium, potassium, magnesium, calcium, manganese, iron, cobalt, nickel, copper, zinc, chlorine, and iodine. Trace elements (light green, pink, or red boxes) occur as occasional enzyme cofactors: boron, fluorine, silicon, arsenic, selenium and bromine, aluminum, gallium, chromium, vanadium, molybdenum, tungsten, and cadmium.
1.5 Fluorides
Fluorine is present on earth only as fluoride, a negatively charged anion that is especially important in dentistry because of its ability to mediate protection from dental caries (Chapter 16, section 16.2.1.). Although metabolites of chlorine and iodine are ubiquitous, few biological products contain fluoride because of its tight hydration shell. The few enzymes that utilize fluoride as a cofactor must overcome an exceptionally high desolvation energy barrier.
The fluoride content of a solution is measured with an electrode made from a mixture of lanthanum and europium fluorides. Lanthanum follows barium in the periodic table and has an atomic number of 57. Lanthanum is also the first of a series of 15 heavy metals before hafnium called lanthanides. Europium is a lanthanide and its atomic number is 63. The mode of action of the fluoride electrode is described in Chapter 16, sect. 16.1.1.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


