Introduction
The aim of this study was to use microcomputed tomography to evaluate the effects on white spot lesions of 3 remineralizing agents compared with artificial saliva (Inonu University, Malatya, Turkey). The agents were GC Tooth Mousse (GC International, Itabashi-ku, Tokyo, Japan), 50-ppm sodium fluoride solution (Inonu University, Malatya, Turkey), and Clinpro 5000 (3M ESPE Dental Products (St Paul, Minn). The experimental and control teeth were stored in artificial saliva.
Methods
Forty-four extracted premolars were divided into 4 groups of 11 teeth each (3 experimental groups and 1 control group). After white spot lesions were created on the teeth, a remineralizing agent was applied. Microcomputed tomography scanning was performed at the following times: T0 (sound enamel), T1 (day 0, when the white spot lesion was formed), T2 (day 15), and T3 (day 30). Volume, depth, surface area, and mineral density changes of the white spot lesions were evaluated at different time points using CTAn software (SkyScan; Bruker, Kontich, Belgium).
Results
GC Tooth Mousse and Clinpro 5000 improved all measurements after 30 days. However, Clinpro 5000 was not as effective in reducing lesion depth as it was in the other parameters. The artificial saliva group and the 50-ppm sodium fluoride solution did not show significant effects in the regression of the white spot lesions at the end of the 30-day experiment.
Conclusions
GC Tooth Mousse and Clinpro 5000 were more effective in remineralization of white spot lesions than sodium fluoride solution and artificial saliva. They can be preferred for use clinically. Microcomputed tomography is a novel and effective method that shows promise in accurately evaluating white spot lesions and remineralization.
Highlights
-
• GC Tooth Mousse is an effective remineralizing agent.
-
• Clinpro 5000 improved some white spot lesion parameters but not lesion depth.
-
• Microcomputed tomography is an effective method to assess white spot lesion changes.
White spot lesions (WSLs), which are subsurface enamel lesions, are undesired side effects of orthodontic therapy with fixed appliances. These lesions can occur around the orthodontic bands and brackets within 4 weeks and cause serious esthetic problems for patients. Many studies have reported that the prevalence and severity of enamel demineralization increases significantly during orthodontic treatment. These lesions prevent an esthetically pleasing smile, which is often the main goal of orthodontic treatment, and they generate an unesthetic appearance on the buccal surfaces of the teeth.
WSLs usually appear in patients with poor oral hygiene, chronic diseases (eg, xerostomia, cerebral palsy), and physical and mental disabilities. These lesions can also appear during orthodontic therapy with fixed appliances when adequate oral hygiene cannot be achieved.
Studies have shown that fixed orthodontic appliances make it difficult to clean teeth and inhibit the natural oromuscular cleansing mechanism. Thus, dental plaque quickly increases around bands and braces. In addition, the pH of this type of plaque is lower than that of normal dental plaque. Patients who are undergoing orthodontic therapy with fixed appliances have a high risk for WSLs. Moreover, fixed orthodontic appliances change the composition of bacterial plaque and also increase the numbers of Streptococcus mutans .
Although preventing WSLs should be the goal of every orthodontist, the incidence of these lesions has been reported to range from 2% to 96% after orthodontic treatment, and the literature lacks information about the effectiveness of remineralizing agents. Therefore, treatment of WSLs using appropriate remineralization agents has great importance. The aim of this study was to evaluate the effects of 4 remineralization agents: GC Tooth Mousse (GC International, Itabashi-ku, Tokyo, Japan), 50-ppm sodium fluoride solution (Inonu University, Malatya, Turkey), Clinpro 5000 (3M ESPE Dental Products, St Paul, Minn), and artificial saliva solution (Inonu University, Malatya, Turkey) using microcomputed tomography (micro-CT) on artificially created WSLs.
Material and methods
Ethical approval (2013/105) was granted by the ethical committee of the Medical Faculty of Inonu University in Malatya, Turkey. All participants and their parents were informed about the study, and written consent was obtained before the experiment. Teeth that were extracted as part of the orthodontic treatment plan were used for the experiment. Power analysis with a 95-μm maximum change for the WSL depth values, an alpha level of 0.05, and a power of 0.8 determined that a minimum of 8 subjects in each group was required (version 3.0.10, G*Power; Franz Faul Universidad, Kiel, Germany). However, 11 teeth were used for each group to reinforce its reliability. Four remineralization agents were used for the experiment ( Table I ).
| Trade name | Chemical composition | Manufacturer, lot number | Application type and duration |
|---|---|---|---|
| GC Tooth Mousse | 10%-20% glycerol, 5%-10% CPP-ACP, 0%-5% D-sorbitol, 0%-2% propylene glycol, 0%-2% silicon dioxide, 0%-2% titanium dioxide | GC International (Itabashi-ku, Tokyo, Japan), 120418M | 1 or 2 times a day, applied for 2 minutes with a finger or cotton |
| Clinpro 5000 | 30%-40% water, 20%-30% crystallizing sorbitol, 10%-20% synthetic amorphous precipitated silica, 1%-10% glycerin, 5%-10% amorphous silica, 1%-5% polyethylene-polypropylene glycol, 1%-5% polyethylene glycol, 1%-2% NaF, <2% seasoning, modified tricalcium phosphate, sodium lauryl sulphate, sodium and carboxymethyl cellulose, sodium saccharin, titanium dioxide | 3M ESPE Dental Products (St Paul, Minn), 12115 | Once a day, before bedtime, applied for 2 minutes |
| 50-ppm sodium fluoride solution | 50-ppm sodium fluoride, deionized water | Inonu University, Faculty of Science, Chemistry Laboratory, Malatya, Turkey | Once per day, waiting in the solution, applied for 2 minutes |
| Artificial saliva | 0.002 g ascorbic acid, 0,030 g glucose, 0.580 g NaCl, 0.170 g CaCl 2 , 0.160 g NH 4 Cl, 1.270 g KCl, 0.160 g NaSCN, 0.330 g of KH 2 PO 4 , 0.200 g urea, 0.340 g Na 2 HPO 4 , mucin 2.700 g, 1000 mL deionized water | Inonu University, Faculty of Science, Chemistry Laboratory, Malatya, Turkey | All day long, waiting in the solution |
Forty-four extracted premolars were randomly divided into 4 groups, each containing 11 teeth. Teeth without restorations, obvious decalcifications, or obvious WSLs were selected as the sample teeth. They were stored at 4°C in 0.01% thymol blue solution until the experimental procedures.
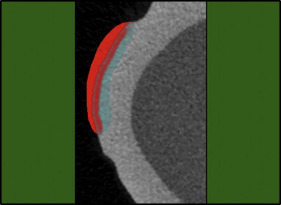
First, the teeth were embedded in acrylic cylinders to standardize the micro-CT scanning. The teeth were then coated with acid-resistant nail varnish (Pinkar, Istanbul, Turkey) leaving a narrow window, approximately 2 × 2 mm in size, on the sound intact surface of the buccal enamel ( Fig 1 ).

In-vitro WSLs were formed on the prepared enamel window using a demineralization solution that included 1 L of deionized water, 0.264 g of monosodium phosphate, 0.244 g of calcium chloride, and 2.86 mL of acetic acid, and the pH was set at 4.4. Each tooth was soaked in this demineralization solution (20 mL) for 96 hours to create the WSLs. Lesion depth, volume, surface area, and mineral density were calculated after the demineralization process using micro-CT (SkyScan 1172; Bruker, Kontich, Belgium).
During the experiment, each tooth was kept in a glass jar in the artificial saliva solution, which was changed once a day. The teeth were kept in artificial saliva solution for 21 hours a day and were placed in the demineralization solution for 2 hours to simulate the oral conditions. For the remaining hour, the treatment procedure was applied to the teeth. The experiment lasted 30 days, and micro-CT scanning was performed at the predemineralization stage (T0) and at days 0 (T1), 15 (T2), and 30 (T3). The following remineralization agents were applied during the experimental period:
- 1.
Artificial saliva group: The specimens in this group were exposed to a daily demineralization-remineralization process. They were kept in artificial saliva for 22 hours and were kept in the demineralization solution for 2 hours in a day to simulate the oral conditions.
- 2.
GC Tooth Mousse group: casein phosphopeptide-amorphous calcium phosphate containing GC Tooth Mousse was applied to the WSL areas for 2 minutes (once a day) with a microbrush. Immediately afterward, the samples were carefully washed with distilled water to remove any excess GC Tooth Mousse. Then the teeth were dried with air and placed in the artificial saliva solution.
- 3.
Clinpro 5000 group: Clinpro 5000, containing 5000 ppm of sodium fluoride, was applied to the lesion areas with a microbrush for 2 minutes. Afterward, the teeth were wiped with distilled water, dried with air, and placed in the artificial saliva solution.
- 4.
50-ppm sodium fluoride group: The 50-ppm sodium fluoride solution was applied to the teeth once a day for 2 minutes. After that, the teeth were washed with distilled water, dried with air, and placed in the artificial saliva solution.
The SkyScan 1172 micro-CT system was used to evaluate the volume (mm 3 ), depth (mm), surface area (mm 2 ), and mineral density (g/cm 3 ) changes in the preformed WSLs. Micro-CT scans were done in the same position using the standard scanning procedure. Each specimen was mounted on the computer-controlled turntable so that the x-ray beam would be perpendicular to the buccal surface of the tooth. Digital sectional images were acquired under the following conditions: 100 mA beam current, 100 kV accelerating voltage, 0.5-mm aluminum and copper filter, 9.9-μm pixel size at 2000 × 2000 resolution dpi, and 360° rotation at the 0.5° step. About 850 to 900 cross-sectional images were taken of each sample with an 11-megapixel camera (Hamamatsu, Tokyo, Japan). The duration of the full scan was about 55 to 60 minutes. The final 3-dimensional (3D) images were evaluated on a 17-in thin-film transistor computer screen by 1 operator (E.K.).
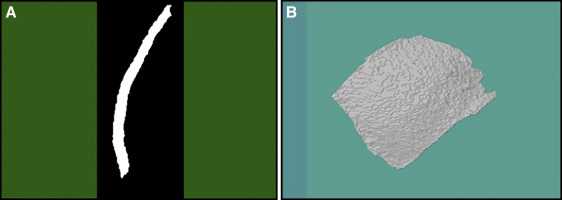
The micro-CT images were analyzed using CTAn (version 1.13.5.1, SkyScan; Bruker) software. The WSLs were separated from the sound enamel around them, the air at the outer surface ( Fig 2 ), and the other tissues in all 3 dimensions using the region of interest function ( Fig 3 ). Then the volume, depth, and surface area of the lesion were calculated with a special task list in the CTAn software. The mineral density of the lesion area was calculated with another task list.
Statistical analysis
Regarding the number of samples in the test groups, nonparametric statistical tests were chosen to evaluate the surface area, volume, depth, and mineral density data for the white spot lesions. Additional ½, ¼, and ¾ deviations were also included in the average and standard deviation values because the tests used were nonparametric.
The Kruskal-Wallis 1-way variant analysis was made to determine whether there was a difference among test groups (α = 0.05). The Bonferroni-corrected Mann-Whitney U test was used to determine the difference between the groups, and the Wilcoxon signed ranked signal test was used to compare the efficacy of the remineralization procedures at the different time points. The data were analyzed using SPSS software (version 20.0; IBM, Armonk, NY).
Results
Table II shows the changes in lesion volume. GC Tooth Mousse and Clinpro 5000 decreased the lesion volumes during the experiment ( P <0.01). The volume changes in the artificial saliva and 50-ppm sodium fluoride groups were statistically insignificant ( P >0.05) ( Table II ). When the remineralization groups were compared at day 30, GC Tooth Mousse had the best remineralization effect on the WSLs.
| Group (n = 11) | Day 0 (T1) | Day 15 (T2) | Day 30 (T3) | Intragroup comparisons | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 1/4 | 1/2 | 3/4 | Mean ± SD | 1/4 | 1/2 | 3/4 | Mean ± SD | 1/4 | 1/2 | 3/4 | T1-T2 | T1-T3 | T2-T3 | |
| Volume (mm 3 ) | |||||||||||||||
| Artificial saliva | 0.52 ± 0.20 a | 0.32 | 0.54 | 0.63 | 0.61 ± 0.18 a | 0.53 | 0.64 | 0.72 | 0.54 ± 0.19 a | 0.34 | 0.58 | 0.66 | ∗ | NS | ∗ |
| GC Tooth Mousse | 0.57 ± 0.15 a | 0.43 | 0.58 | 0.64 | 0.51 ± 0.11 a | 0.40 | 0.47 | 0.60 | 0.45 ± 0.11 a | 0.37 | 0.46 | 0.55 | ∗ | ∗ | ∗ |
| Clinpro 5000 | 0.65 ± 0.99 a | 0.55 | 0.63 | 0.74 | 0.57 ± 0.15 a | 0.45 | 0.62 | 0.70 | 0.52 ± 0.16 a | 0.40 | 0.56 | 0.69 | ∗ | ∗ | ∗ |
| 50 -ppm NaF | 0.65 ± 0.18 a | 0.54 | 0.66 | 0.82 | 0.67 ± 0.20 b | 0.52 | 0.70 | 0.84 | 0.65 ± 0.20 b | 0.51 | 0.68 | 0.81 | NS | NS | NS |
| Depth (mm) | |||||||||||||||
| Artificial saliva | 0.10 ± 0.03 a | 0.08 | 0.11 | 0.13 | 0.12 ± 0.02 a | 0.01 | 0.12 | 0.14 | 0.11 ± 0.02 a | 0.09 | 0.12 | 0.13 | ∗ | † | ∗ |
| GC Tooth Mousse | 0.11 ± 0.02 a | 0.10 | 0.11 | 0.12 | 0.10 ± 0.01 a | 0.09 | 0.10 | 0.11 | 0.09 ± 0.01 a | 0.09 | 0.09 | 0.10 | ∗ | ∗ | ∗ |
| Clinpro 5000 | 0.12 ± 0.01 a | 0.11 | 0.12 | 0.13 | 0.11 ± 0.01 a | 0.10 | 0.12 | 0.14 | 0.11 ± 0.02 a | 0.09 | 0.12 | 0.14 | ∗ | NS | NS |
| 50-ppm NaF | 0.13 ± 0.01 a | 0.11 | 0.13 | 0.14 | 0.14 ± 0.02 ab | 0.12 | 0.14 | 0.16 | 0.13 ± 0.02 ab | 0.12 | 0.14 | 0.16 | ∗ | NS | ∗ |
| Area (mm 2 ) | |||||||||||||||
| Artificial saliva | 13.86 ± 1.82 a | 13.15 | 14.22 | 14.67 | 14.08 ± 1.98 a | 13.39 | 14.19 | 14.99 | 13.66 ± 2.24 a | 10.77 | 14.48 | 14.88 | NS | NS | NS |
| GC Tooth Mousse | 15.02 ± 3.10 a | 11.59 | 15.02 | 17.08 | 14.40 ± 3.10 a | 10.98 | 14.97 | 16.62 | 13.99 ± 2.59 a | 11.47 | 14.98 | 15.66 | ∗ | ∗ | NS |
| Clinpro 5000 | 15.03 ± 1.24 a | 13.81 | 15.47 | 16.10 | 13.87 ± 0.88 a | 13.15 | 13.64 | 14.82 | 13.67 ± 0.88 a | 13.07 | 13.32 | 14.26 | ∗ | ∗ | NS |
| 50-ppm NaF | 14.37 ± 2.69 a | 13.59 | 15.01 | 16.65 | 13.63 ± 2.54 a | 12.85 | 14.50 | 15.01 | 13.60 ± 2.50 a | 12.42 | 14.43 | 15.22 | ∗ | ∗ | NS |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


