Bone regeneration using cancellous cellular marrow grafts has been the mainstay for oral and maxillofacial surgeons simply because they produce the most bone in the correct arch form and are best able to support functional needs. Even though most oral and maxillofacial surgeons today use such cancellous cellular marrow grafts, many other head and neck surgeons use microvascular fibula grafts. Each has its advocates and its own set of advantages and disadvantages.
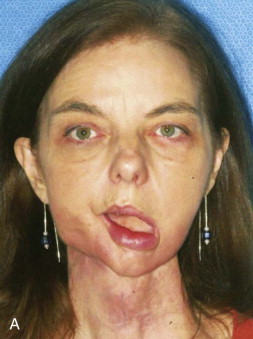
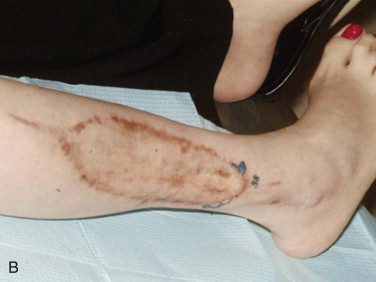
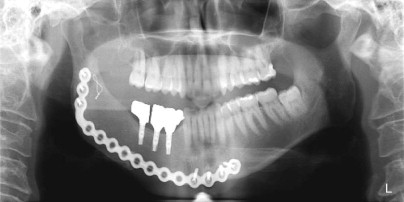
A free vascular fibula graft has the advantage of being useful for immediate reconstruction in the presence of an oral communication and is capable of bringing soft tissue along with bone. However, the fibula is too small and straight to conform to the arch form of the mandible or maxilla, and because of its strictly cortical nature and low profile, it does not hold dental implants well after functional loading ( Fig. 66-1 ). In addition, the length of surgery, the need for postoperative intensive care unit (ICU) management, the morbidity of leg and foot paresthesia ( Fig. 66-2 ), and weakness of the great toe have always been its major disadvantages. It is also a graft that may be lost as a result of failure of the vascular anastomosis.
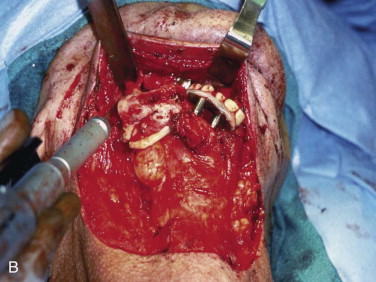
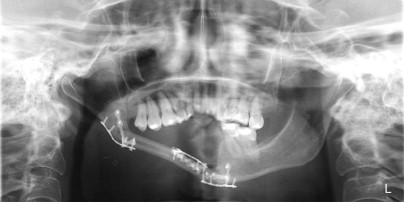
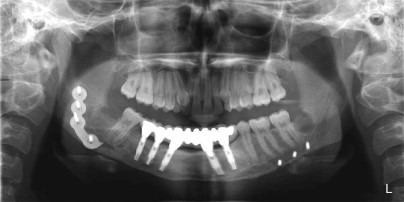
A cancellous marrow graft, in contrast, has the disadvantage of being prone to infection because of oral communications and is unable to be used for the reconstruction of soft tissue loss at the same time, thus necessitating delayed, yet definitive reconstruction. Its advantages are that it predictably regenerates bone of greater height, width, and arch form than a fibula graft does ( Fig. 66-3 ) and it has an excellent track record of implant osseointegration in which implants can be placed into more ideal position for functional loading ( Fig. 66-4 ). Cancellous marrow grafting is a less lengthy surgical procedure, does not usually require postoperative ICU stay, and is associated with much less and a more reversible donor site morbidity.
Biology of Bone Regeneration Grafts
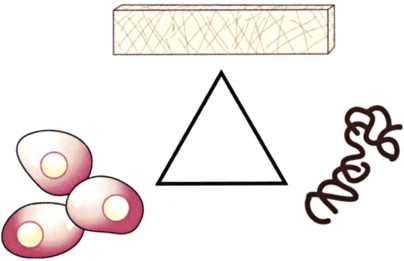
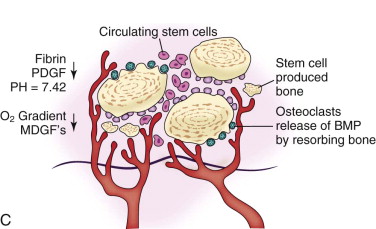
Whereas in a microvascular bone transfer a preformed bone is moved from one anatomic site to another site (the leg to the jaws in this case), a cancellous cellular marrow graft regenerates a new remodeling bone that is sized and shaped to the needs of the recipient site. Regeneration of bone relies on the classic tissue-engineering triangle of cells (in this case mesenchymal stem cells and osteoprogenitor cells), a signal (growth factors such as bone morphogenetic protein [BMP], insulin-like growth factor [IGF], and transforming growth factor-β [TGF-β]), and a matrix (a scaffold) for bone development ( Fig. 66-5 ). An autogenous cancellous marrow graft contains all three components of this tissue-engineering triangle, which is why it is referred to as the “gold standard.” At the time of this writing, the three portions of this triangle have been put together separately to regenerate functionally useful bone in patients without using any autogenous bone source. This new biotechnology is discussed later in this chapter.
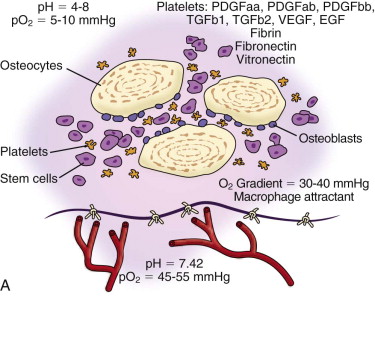
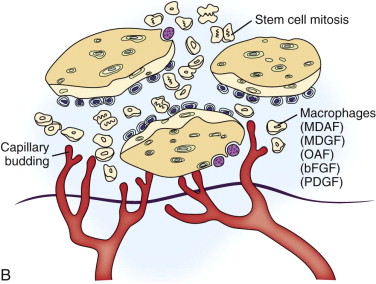
In the routinely used cancellous marrow graft, trabecular bone from the donor site that has surface osteoblasts, osteoprogenitor cells, and mesenchymal stem cells ( Fig. 66-6 ) is placed into the recipient site along with the blood clot inherent in all wounds. The platelets within the blood clot degranulate and liberate seven growth factors (platelet-derived growth factors AA, BB, and AB; TGF-β1 and TGF-β2, vascular endothelial growth factor, and epithelial growth factor) into the local environment ( Fig. 66-7, A ). These growth factors initiate the proliferation of osteoprogenitor cells, osteoblasts, and mesenchymal stem cells, as well as capillary ingrowth. After 7 days the platelets are depleted, but by that time, wound-healing macrophages have migrated into the hypoxic environment of the wound and continue the secretion of growth factors ( Fig. 66-7, B ). By days 14 to 20 the graft is completely revascularized and osteoid production has started ( Fig. 66-7, C ). Because of the fragile state of the graft for these first 21 days, graft site immobility is crucial and can be achieved with maxillo-mandibular fixation, titanium plates and mesh, membranes, and other means, depending on the size and location of the graft.
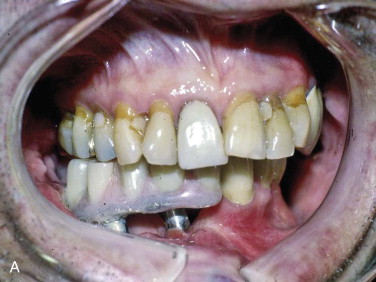
As a result of the nutrients derived from capillary ingrowth, significant osteoid production occurs, consolidates the graft into a single unit, and fuses it to the host bone from the third to the sixth weeks. At this time any fixation or stabilizing device can be released and light function begun. After 6 weeks the graft is set but contains immature bone that will undergo the normal physiologic resorption–new bone apposition–remodeling cycle ( Fig. 66-8 ) to create a more mature and mineralized bone, which by 6 months can receive dental implants or support conventional dentures ( Fig. 66-9 ).
Techniques for Grafting Mandibular Continuity Defects
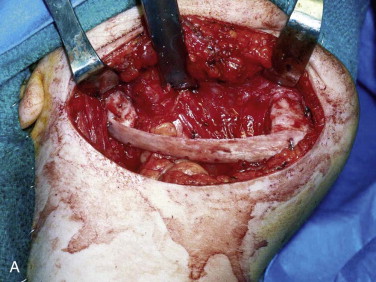
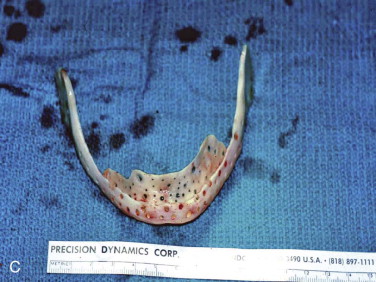
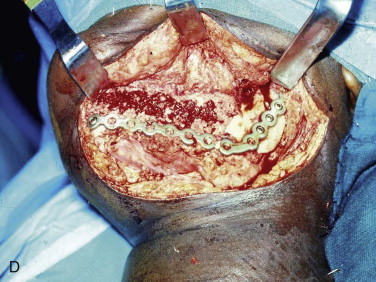
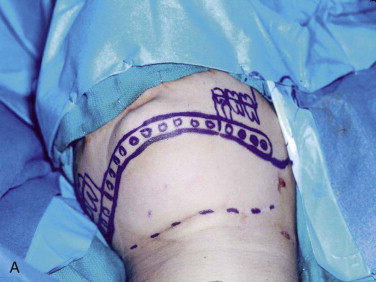
The three important clinical scenarios involved in reconstructing mandibular continuity defects are (1) a hemimandibular defect with a proximal and distal native bone edge, (2) a hemimandibular defect with a missing condyle but with a distal native bone edge, and (3) a defect that may be of varying size and crosses the midline but has a right and left proximal bone edge. The commonality of each case is that autogenous cancellous marrow usually harvested from the posterior ilium is the graft material and that crib containment must be planned. The containment may be a crib of either various allogeneic bone segments (split ribs, ilium forms, or hollowed-out mandibular cribs), titanium mesh cribs, or titanium plates with soft tissue sutured directly to the plate or used together with allogeneic bone cribs or titanium mesh cribs.
The variability in each case involves the location of the defect and the quality and quantity of the soft tissue. In patients with soft tissue deficiency because of tumor surgery or radiation-induced fibrosis, it is best to add soft tissue with a flap suited to the size of the defect 3 months in advance of the bone graft. In patients who have received radiation therapy, the site should be improved by using the standard protocol of hyperbaric oxygen: 30 sessions of 100% oxygen at a pressure of 2.4 atm for 90 minutes each session.
Hemimandibular Defect with a Proximal and Distal Bone Edge
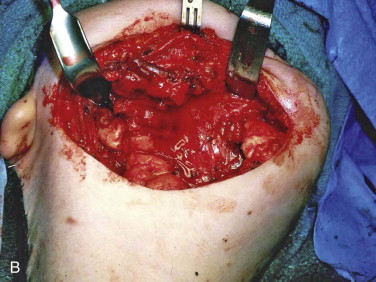
The incision is best placed in the neck within a natural skin fold, within a previous surgical scar, or parallel to the inferior border of the mandible and 3 cm below it ( Fig. 66-10, A ). The plane of dissection to the mandible should be deep to the superficial layer of the deep cervical fascia to preserve the marginal mandibular branch of the facial nerve. The periosteum of each native bone edge needs to be thoroughly reflected for 3 to 4 cm from the edge of the defect ( Fig. 66-10, B ). If a titanium plate is in place from the ablative surgery, it should be removed and the band of plate scar excised. To complete development of the tissue bed, scar tissue needs to be released to the level of the submucosa without perforation into the mouth. This may be facilitated by either palpating the maxillary teeth through the mucosa to gain an appreciation of the thickness of the tissue or by placing a gloved finger in the mouth to guide the dissection and then changing the glove when this maneuver is completed.
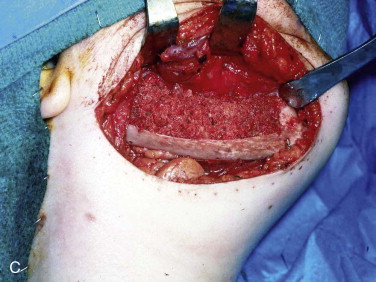
The graft material is then compacted with amalgam pluggers or Penfield bone packers beginning at each bone segment and working toward the center while being sure to pack the graft material to the height of the dissected recipient tissue ( Fig. 66-10, C ). Whatever crib is used, rigid titanium plate, titanium mesh, allogeneic bone, or combinations, the surgeon should suture the fascia of the neck to the crib so that the graft does not become displaced into the neck. This is followed by closure of the periosteum, fascia/platysma, subcutaneous tissue, and skin. The author usually places a non-suction drain (Penrose drain) between the periosteum and fascia/platysma, followed by a pressure dressing ( Fig. 66-10, D ).
Hemimandibular Defect with a Missing Condyle
This defect is grafted with the techniques described for a hemimandibular defect with both proximal and distal bone segments except for the development of a condylar articulation. There are several ways that a condylar articulation can be created. The first is to also harvest a rib for use in the costochondral articulation and as part of the containment crib if it is secured to the inferior border ( Fig. 66-11, A ). The second is to use an allogeneic mandibulare crib in which the condyle is hollowed out ( Fig. 66-11, B ). The cancellous cellular marrow graft is then packed into the hollowed-out mandible, as well as the hollowed-out condyle ( Fig. 66-11, C ). A third is to use a titanium plate with an allogeneic ramus and condyle affixed to it, which is once again hollowed out to receive cancellous cellular marrow ( Fig. 66-11, D ). A fourth is to use a titanium plate with a titanium condyle and place the cancellous marrow lingual to the plate but no higher than the mid-ramus ( Fig. 66-11, E ). The titanium condyle would be the permanent articulation and the bone graft itself would be the reconstruction that would allow dental rehabilitation. Because of the past poor experience with total temporomandibular joint (TMJ) prostheses in patients with temporomandibular dysfunction (TMD), this approach may be thought to be risky. However, the use of titanium condyles that articulate against a native disc or soft tissue interface in the fossa in tumor patients rather than TMD patients has a proven track record of stable and uncomplicated function and is the author’s preferred approach in this situation.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses