15
Maxillary Anesthesia II: Complementary Anesthesia of the Palate
Introduction
Clinical experience shows that the buccal infiltrative technique applied via the buccal area does not usually anesthetize the palate, therefore some interventions must be completed with anesthesia of the palate, hence the concept of complementary anesthesia of the palate. In any case it is important to remember that the palate is innervated and that the fibromucosa, periosteum, and bone are innervated by two nerve trunks:
- The nasopalatine nerve, which emerges from the incisive foramen, supplies the area of the palate at the incisive papilla and the areas close to the central and lateral incisors.
- The greater palatine nerve, which emerges at the back part of the palate via the greater palatine foramen, supplies the palate in the canine, premolar, and molar areas on each side as far as the raphe.
This chapter reviews three techniques for anesthetizing the nerve trunks of the palate:
- Nasopalatine nerve block and its intranasal variant.
- Greater palatine nerve block and its variant in the area of cross‐innervation extending from the first premolar to the lateral incisor.
- Transpapillary technique for children.
Some of the characteristics of the techniques used to anesthetize the palate are now reviewed.
The Nasopalatine Nerve Innervates Less than Previously Thought
Many texts continue to attribute a much greater area of innervation to the nasopalatine nerve than it actually has (Bennett 1984; Jastak et al. 1995; Malamed 2004). Clinical research has shown that the area between the lateral incisor and the first premolar is an area of cross‐innervation where the main supply is from the greater palatine nerve. Thus, the greater palatine nerve supplies the first premolar in 95% of cases, the canine in 75% of cases, and the lateral incisor in 50% (see Table 2.1, Chapter 2).
The Potency of the Anesthetic is not Important
The potency of the anesthetic solution, according to the type and concentration of the anesthetic and/or vasoconstrictor, is not important for ensuring anesthesia of these nerves via the palate because they are superficial and not covered by periosteum or cortical bone, therefore little anesthetic is required to anesthetize the area.
Anesthesia of the Palate Without Complementary Palatal Anesthesia
Clinical studies show that use of potent solutions such as articaine 4% with epinephrine 1:100 000 (10 μg/ml) (A‐100) in extractions in the area of the molars and premolars principally is successful in 94% of cases with the buccal infiltrative technique. This approach does not require complementary palatal anesthetic, although patients may complain of mild discomfort in the palate. However, when the standard solution is used (lidocaine 2% with epinephrine 1:100 000) (L‐100), the success rate falls to 55% (Table 15.1). Some authors recommend waiting longer (7–9 minutes) for the solution to reach the palate. In addition, this approach is more effective in anterior teeth than in posterior teeth (Kumaresan et al. 2015).
Studies based on magnetic resonance imaging do not reveal diffusion of the anesthetic solution from the buccal area to the palate (Özec et al. 2010); however, experimental animal studies did find concentrations of anesthetic solution in the bone and the mucous membrane of the palate. These are greater with A‐100 than with L‐100 (Al‐Mahalawy et al. 2018). Reported findings are contradictory.
Table 15.1 Success (%) of extraction of maxillary molars (M) and premolars (PM) after buccal infiltration with a potent anesthetic solution (A‐100) or standard solution (L‐100 or L‐80) and no complementary palatal anesthetic.
| Solution | Palatal anesthetic | Success (%) | |||
|---|---|---|---|---|---|
| Reference | Sample size | Tooth | ml/LAS/time (min) | ml/LAS | (proportion) |
| No complementary anesthesia with A‐100 | |||||
| Uckan et al. (2006) | 53 | — | 2.0/A‐100/5′ | No | 96% (51/53) |
| Fan et al. (2009) | 71 | — | 1.7/A‐100/5′ | No | 90% (64/71) |
| Lima‐Junior et al. (2009) | 50 | 3rdM | 1.8/A‐100/10′ | No | 94% (47/50) |
| Lima‐Junior et al. (2013) | 15 | 3rdM | 1.8/A‐100/5′ | No | 100% (15/15) |
| Somuri et al. (2013) | 30 | PM | 1.7/A‐100/−− | No | 90% (27/30) |
| Darawade et al. (2014) | 50 | PM | 0.8/A‐100/— | No | 100% (50/50) |
| Sharma et al. (2014) | 80 | M, PM | 0.9/A‐100/— | No | 94% (75/80) |
| Kandasamy et al. (2015) | 116 | — | 1.7/A‐100/10′ | No | 93% (106/116) |
| Bataineh and Al‐Sabri (2017) | 48 | M, PM | 1.8/A‐100/5′ | No | 92% (44/48) |
| Majid and Ahmed (2018) | 28 | M | 1.8/A‐100/10′ | No | 86% (24/28) |
| Bataineh et al. (2019) | 50 | I, PM, M | 1.3/A‐87/12′ | No | 94% (47/50) |
| Mean | 94% | ||||
| No complementary anesthesia with L‐100 | |||||
| Badcock et al. (2007) | 51 | 3rdM | 2.2/L‐80/5′ | No | 86% (44/51) |
| Lassemi et al. (2008) | 30 | IC | 1.8/L‐80/6′ | No | 77% (23/30) |
| Darawade et al. (2014) | 50 | PM | 1.3/L‐100/— | No | 2% (1/50) |
| Kandasamy et al. (2015) | 111 | — | 1.7/L‐80/10′ | No | 1% (1/111) |
| Kumaresan et al. (2015) | 25 | M | 1.5/L‐80/7–9′ | No | 52% (13/25) |
| Majid and Ahmed (2018) | 28 | M | 3.6/L‐100/10′ | No | 86% (24/28) |
| Bataineh et al. (2019) | 50 | I, PM, M | 1.3/L‐75/10′ | No | 96% (48/50) |
| Mean | 55% | ||||
N, sample size; ml, milliliters injected; LAS, local anesthetic solution; A‐100, articaine 4% + epinephrine 1:100 000 (10 μg/ml); A‐87, articaine 4% + epinephrine 1:87 000 (11.5 μg/ml); L‐100, lidocaine 2% + epinephrine 1:100 000 (10 μg/ml); L‐80, lidocaine 2% + epinephrine 1:80 000 (12.5 μg/ml); L‐75, lidocaine 2% + epinephrine 1:75 000 (13.3 μg/ml); 5 is 5 minutes waiting time after administering the anesthetic.
Nevertheless, when complementary palatal anesthetic is used, irrespective of whether it is with A‐100 or L‐100, the success rate is practically 100% (Table 15.2).These successes in the third maxillary molars may be due to the fact that extraction at this level is relatively simple and rapid (less than 1 minute in most cases) and that the depth of anesthesia necessary for these extractions is lower than that needed in other procedures, such as endodontic procedures (Badcock et al. 2007). However, extraction of first molars may take longer (around 4 minutes) and be more complicated, although it is also successful, and extractions with complementary palatal anesthetic are more comfortable for the patient (Majid and Ahmed 2018).
Indications
These techniques are not aimed at achieving pulpal anesthesia (Hicks et al. 1995), but rather at providing complementary anesthesia of the palate in the following cases:
- Dental surgery and extractions (Roberts and Sowray 1987; Evers and Haegerstam 1981).
- Reinforcing pulpal anesthesia after a buccal infiltration in healthy teeth (Guglielmo et al. 2011) or in the case of irreversible acute pulpitis (Aggarwal et al. 2011; Ulusoy and Alacam 2014; Askari et al. 2016), given that in teeth with palatal roots the percentage of pulpal anesthesia increases.
- Scaling and root planing.
- Subgingival preparations in the palate:
- Restorations.
- Restorations by cervical caries.
- Placement of a retraction cord.
- Gingival retraction in the palate.
- Insertion of subgingival matrix bands at the level of the palate (Malamed 2004).
- Placement of clamps for a dental dam that penetrate or pinch the mucous membrane of the palate.
Table 15.2 Success (%) of extraction of maxillary molars (M) and premolars (PM) after buccal infiltration, based on a potent anesthetic (A‐100) or the standard solution (L‐100 or L‐80) with complementary palatal anesthesia.
| Solution | Palatal anesthetic | Success (%) | |||
|---|---|---|---|---|---|
| Reference | Sample size | Tooth | ml/LAS/time (min) | ml/LAS | (proportion) |
| Complementary anesthesia with L‐100 | |||||
| Uckan et al. (2006) | 53 | — | 1.8/L‐100/5′ | 0.5/L‐100 | 98% (52/53) |
| Lassemi et al. (2008) | 30 | IC | —/L‐80/6′ | —/L80 | 100% (30/30) |
| Somuri et al. (2013) | 30 | PM | 1.8/L‐100/— | 0.25/L‐100 | 100% (30/30) |
| Sharma et al. (2014) | 80 | M, PM | 1.8/L‐100/— | —/L‐100 | 100% (80/80) |
| Kumaresan et al. (2015) | 25 | M | 1.5/L‐80/2′ | 0.3/L‐80 | 100% (25/25) |
| Bataineh et al. (2019) | 55 | I, PM, M | 0.9/L‐75/7′ | 0.2/L‐75 | 100%(55/55) |
| Mean | 99.6% ≈ 100% | ||||
| Complementary anesthesia with A‐100 | |||||
| Fan et al. (2009) | 71 | — | 1.7/A‐100/5′ | 0.4/A‐100 | 95% (67/71) |
| Lima‐Junior et al. (2009) | 100 | 3M | 1.8/A‐100/5–10′ | —/A‐100 | 100% (100/100) |
| Majid and Ahmed (2018) | 28 | M | 1.8/A‐100/10′ | 0.2/A‐100 | 100% (28/28) |
| Mean | 98.3 ≈ 100% | ||||
N, sample size; ml, milliliters injected; LAS, local anesthetic solution; A‐100, articaine 4% + epinephrine 1:100 000 (10 μg/ml); L‐100, lidocaine 2% + epinephrine 1:100 000 (10 μg/ml); L‐80, lidocaine 2% + epinephrine 1:80 000 (12.5 μg/ml); L‐75, lidocaine 2% + epinephrine 1:75 000 (13.3 μg/ml); 5 is 5 minutes waiting time after administering the anesthetic.
Methods for Reducing Pain in Palatal Techniques
Clinical observation tells us that injections into the palate are the most painful (Kramp et al. 1999; Wahl et al. 2001; Primosch and Brooks 2002). Clinical studies confirm this observation (Annexes 19 and 23). This is because the palatal fibromucosa is formed by dense fibers, with little subcutaneous tissue, and is firmly attached to the periosteum. Consequently, elasticity is minimal, the ability to spread after administration is limited, and the technique is painful (Gill and Orr II 1979; Keller 1985; Kreider et al. 2001; Bhalla et al. 2009). In addition, the anterior part of the palate is known to be somewhat more painful than the posterior part, precisely because the fibromucosa is more firmly attached (Meechan et al. 2005; Özec et al. 2010).
Several methods are used to prevent or at least minimize the pain induced by injection into the palate. However, as expected, when several methods are available and no consensus has been reached on which is best, this is because neither is completely satisfactory.
Topical Anesthesia
Topical anesthesia is poorly effective in the palate, as shown in clinical trials (Annex 19). These poor results are due not only to previous comments (fibromucosa firmly attached to the periosteum), but also to the fact that because the mucous membrane of the palate is more keratinized, its permeability is lower than that of the rest of the oral mucous membrane (Lesch et al. 1989), leading to reduced penetration of topical anesthetic.
In any case, topical anesthesia reduces the average perception of pain compared with placebo, although the differences are not significant.
Pressure Techniques
Conventional methods (Malamed 2004)
These techniques involve applying firm and continuous pressure with a cotton swab (e.g. those used for application of topical anesthesia) on the surface of the mucous membrane of the palate to be injected for 20–30 seconds before insertion. The pressure should be sufficiently firm for the mucous membrane to change from its usual pink color to a white or pale color owing to the blanching caused by the pressure applied. The injection should be made very close to the head of the swab, and pressure should be maintained throughout the injection.
“Press and roll” technique (Kravitz 2006)
Inform the patient that you are about to place topical anesthetic on the palate (optional, although it does reassure the patient) and that he/she will feel considerable pressure on the palate during the maneuver.
- Dry the mucous membrane with a gauze.
- Cover the area with topical anesthesia and leave it for about 1–2 minutes (optional).
- Apply pressure on the area of the palate to be injected with the end of the shaft of a dental mirror.
- Insert the needle while pressing it against the shaft of the mirror, almost pushing it below the shaft and at the same time press with the shaft.
- Inject a few drops of anesthetic and then turn the shaft of the mirror toward the needle. Inject for only 3–5 seconds. This maneuver leaves the mucous membrane white or pale owing to the blanching induced by the pressure (Figure 15.1).
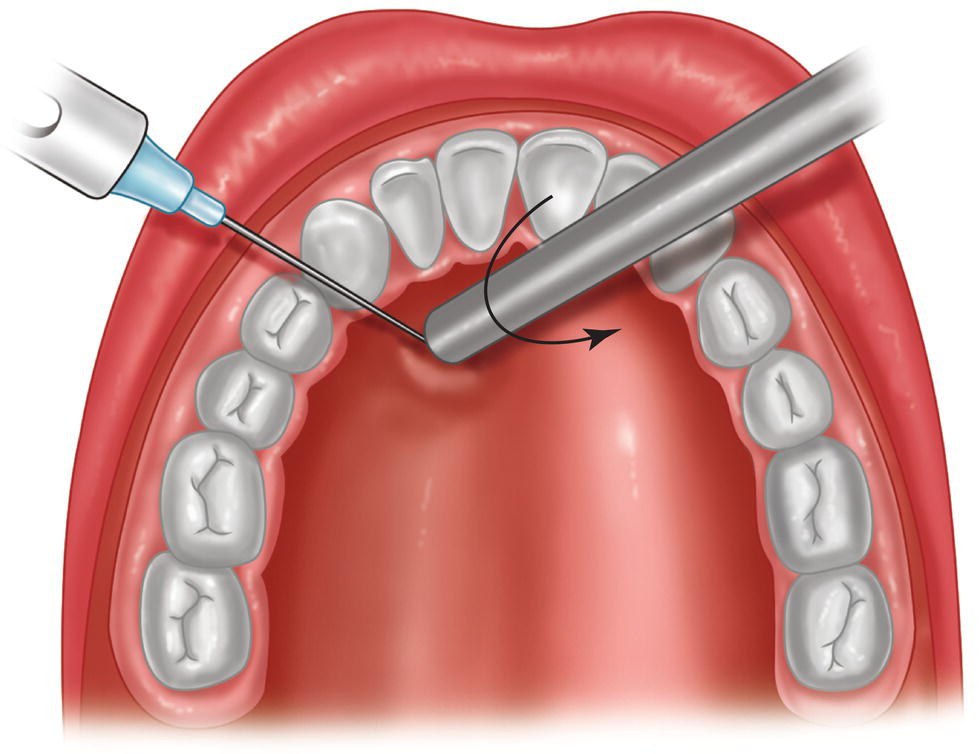
Figure 15.1 Needle inserted under the tip of the shaft. The exact point at which a few drops are injected and the shaft turns toward the needle while applying pressure.
Source: Redrawn from Kravitz (2006).
- Remove the needle, remove the shaft of the mirror, and wait a minute for the few drops of anesthetic solution to take effect and the soft tissues at the injection site to become anesthetized.
- After aspirating, reinsert the needle at the same site to inject a larger quantity of anesthesia (±1/8 of a cartridge or 0.25 ml) slowly (7–10 seconds) and wait a further 30 seconds. A small amount of anesthetic is preferred to a large amount to reinforce the previous amount and ensure that the palate starts to become anesthetized.
This technique is fairly fast without topical anesthetic.
Pressure maneuvers act by activating the gate control system in the trigeminal nerve nuclei. This partially inhibits the passage of pain stimuli to higher pathways, thus helping the stimulus to remain below the pain threshold (Melzack and Wall 1965; Dubner 1978) (see Chapter 11).
Topical Cooling
Cold reduces the velocity of nerve conduction, which ceases when the temperature falls from 10 to 0 °C (Harbert 1989), thus inducing anesthesia. Topical cooling techniques are used before injection of palatal local anesthesia to ensure that the procedure is as painless as possible. The only contraindication would be in patients who cannot tolerate cold (Harbert 1989).
There are three different approaches: (i) old cold aerosols, like ethyl chloride; (ii) refrigerants, only used on the palate (Duncan et al. 1992; Kosaraju and Vandewalle 2009; Wiswall et al. 2014); and (iii) topical ice. All these methods are explained in Chapter 12.
Periodontal Ligament Technique
This technique was first proposed by Dr. Barry McArdle as being almost painless (McArdle 1997
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


