Injury to peripheral branches of the trigeminal nerve can arise from a wide variety of oral and maxillofacial surgical procedures or injuries, including dentoalveolar surgery, placement of dental implants, endodontic therapy, orthognathic surgical procedures, removal of benign or malignant tumors, and local anesthetic injections, as well as be a direct consequence of maxillofacial trauma or surgical interventions for repair of facial trauma.
The most commonly injured nerves in oral and maxillofacial surgery include the lingual nerve (LN) and inferior alveolar nerve (IAN) ( Fig. 29-1 ). Injuries to these nerves produce decreased or lost sensation or painful sensations that interfere with appropriate sensory perception by the central nervous system (CNS). Loss or aberration of orofacial sensory input is detrimental to patients because of negative effects on speech, taste, swallowing, ability to maintain food and liquid competence, social interactions, playing of wind musical instruments, and pain perception. The majority of these injuries result in sensory changes that are temporary in nature and recover spontaneously with time. Although all nerves respond to injury with stereotypic pathophysiologic responses, genetic, hormonal, anatomic, physiologic, behavioral, or other factors may influence any individual nerve’s recovery. Four factors are known to affect the rate and degree of peripheral sensory nerve recovery following injury age, state of general health, location of the injury, and type of injury.

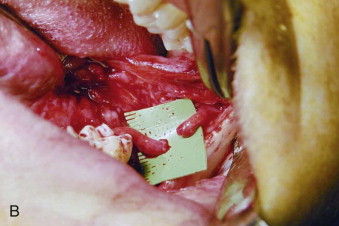
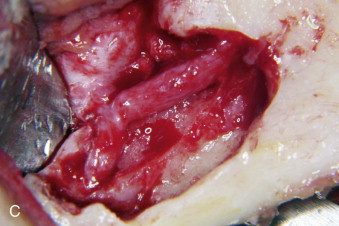
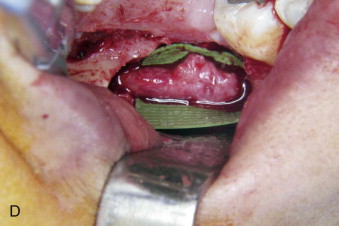
The timing of surgical repair of a nerve injury remains a controversy. A consensus was reached and reported in 1992 by Alling and colleagues. Although there remains little scientific evidence to support these recommendations regarding the treatment of nerve injury, the timing of nerve repair surgery was originally based on the extensive experience of Seddon during and after World War II. During the ensuing years, the extensive clinical experience of oral and maxillofacial surgeons has validated the concepts of timing that were previously merely speculative. Based on this experience, Meyer and Ruggiero proposed specific timing guidelines. Because it is impossible to conduct valid prospective randomized clinical trials to compare early versus late repair, the surgeon is relegated to relying on retrospective cohort studies. This makes it difficult to know whether patients who undergo early repair would have improved (and to what degree) without surgical intervention.
Microsurgical operative management may be the most effective approach to restoring function in the subset of patients in whom significant LN or IAN sensory dysfunction has failed to resolve spontaneously after a reasonable interval of clinical observation. A number of outcome studies have explored the effects of microsurgical repair of the LN and IAN. Multiple studies have suggested no association between delayed repair and neurosensory outcome, whereas others, including our own work, strongly suggest improved outcomes with early repair. This controversy is primarily the result of the literature containing small case series with nonstandardized methods for evaluating outcomes, which makes comparison between studies problematic and their outcomes difficult to evaluate.
We retrospectively reviewed 222 LN and 186 IAN injuries that were observed for at least 1 year and demonstrated that microsurgical repair of LN and IAN injuries can result in sensory and functional improvement in patients who have surgical indications as determined by the history and standardized neurosensory testing (NST). The majority of operated patients do regain acceptable sensation and associated function as classified by the Medical Research Council (MRC) scale ( Table 29-1 ). Relief of pain is also frequently a welcome benefit of surgical treatment. Microsurgical repair of an injured LN or IAN is a valid treatment for many patients. In our studies, the likelihood of recovery following nerve repair decreases progressively with time after injury and with increasing age of the patient.
| GRADE * | DESCRIPTION |
|---|---|
| S0 | No sensation |
| S1 | Deep cutaneous pain in an autonomous zone |
| S2 | Some superficial pain and touch sensation |
| S2+ | Superficial pain and touch sensation plus hyperesthesia |
| S3 | Superficial pain and touch sensation without hyperesthesia; static 2-point discrimination >15 mm |
| S3+ | Same as S3 with good stimulus localization and static 2-point discrimination of 7-15 mm |
| S4 | Same as S3 and static 2-point discrimination of 2-6 mm |
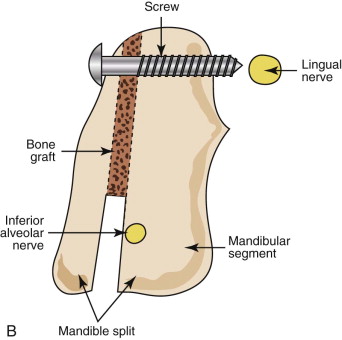
Injuries to the IAN as a result of mandibular sagittal split ramus osteotomy (SSRO) deserve special attention and are distinguished by their anatomic and surgical complexity in comparison to third molar surgery. Since its introduction by Schuchardt and modification and popularization by Trauner and Obwegeser, SSRO has undergone multiple surgical refinements and instrument/equipment modifications to improve its results. SSRO is currently the most widely accepted method for surgical correction of mandibular skeletal abnormalities. Despite the versatility and numerous advantages of this procedure, neurosensory disturbances are common and are related to the surgical anatomy involved in this operation ( Fig. 29-2 ). Postoperatively, transient or temporary changes in sensory function of the peripheral trigeminal nerve branches, most often the IAN but also including the LN, are an expected part of the patient’s recovery as a result of these nerves’ intimate proximity to the surgical site.
This chapter reviews the basics of microsurgical repair of the IAN and LN. Readers should refer to Suggested Readings for more in-depth information.
Incidence/Etiopathogenesis
Although estimates vary, it is supported within the literature that a small number of patients who have sustained nerve injury experience permanent neurosensory dysfunction (NSD). The rate of permanent injury to the LN from third molar surgery ranges between 0.04% and 0.6%, whereas for the IAN it ranges between 0.1% and 1%. The incidence of persistent nerve impairment of the IAN 1 year after SSRO surgery in a recent systematic review was reported to be 12.8%. Spontaneous recovery of IAN function has been documented following SSRO, with the greatest improvement having been found in the first 3 months after injury. Numerous reports document sensory changes in the IAN after SSRO, but few have explored the incidence of temporary or permanent LN sensory alterations.
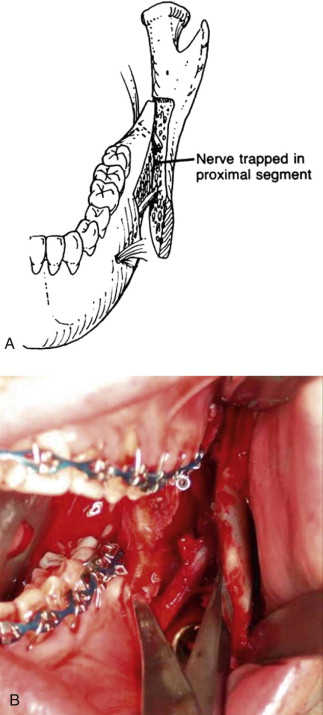
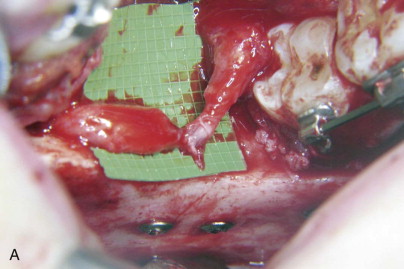
Fewer reports have mentioned LN changes after SSRO. Damage to the LN can be incapacitating to patients, who may experience tongue biting, difficulty masticating or tooth brushing, impaired phonation or swallowing, and loss or altered sense of taste because of paresthesia or dysesthesia in the tongue or lingual gingiva. Risk factors for LN injury during SSRO have not been emphasized previously. In our experience, the common mechanism of injury to the LN during SSRO occurs during the placement of bicortical superior border internal fixation screws. This may occur secondary to perforation of the lingual cortical bone of the mandible by the drill and inadvertent penetration of the unprotected LN or, less commonly, secondary to impalement of the LN by a fixation screw that extends beyond the limits of the bone ( Fig. 29-3 ).
Pathologic Anatomy
Anatomic drawings and descriptions that appear in textbooks are merely representations of average locations of the various human structures determined from observing literally hundreds of cadaver dissections. Therefore, the skeletal and soft tissue structures of the human head and neck may have significant variations from the accepted anatomic norms. The anatomy of the trigeminal nerve, as described in standard texts, is complex, but not necessarily accurate for a given patient. Perhaps variation in the position of the nerve or associated musculoskeletal structures contributes to the incidence of nerve injuries despite correct surgical techniques. In the largest known cadaver study, Behnia and associates looked at the anatomy of the LN in 669 nerves from 430 fresh cadavers. Measurements on each cadaver were made with a micrometer caliper to determine the horizontal and vertical position of the LN in the lower third molar region. In 94 cases (14.0%), the nerve was above the lingual crest, and in 1 case (0.15%), the nerve was in the retromolar pad region. In the remaining 574 cases (85.8%), the mean horizontal and vertical distances of the nerve to the lingual plate and lingual crest were 2.1 ± 1.1 mm and 3.0 ± 0.4 mm (range, 1.7 to 4.0 mm), respectively. In 149 cases (22.3%), the nerve was in direct contact with the lingual plate of the alveolar process. This study confirms the variable anatomy of the trigeminal nerve in the maxillofacial region.
Similarly, the position of the IAN has great variability. Although the nerve is known to traverse along an interosseous path from the mandibular foramen on the medial mandibular ramus to the mental foramen on the lateral mandibular body, its position within the mandible can have significant interosseous variation superoinferiorly or mediolaterally (or both) and in its relationship to the mandibular molar and premolar teeth ( Fig. 29-4 ). In addition, the position of the nerve changes relative to the alveolar crest if the edentulous mandible undergoes resorption with age.
Pathologic Anatomy
Anatomic drawings and descriptions that appear in textbooks are merely representations of average locations of the various human structures determined from observing literally hundreds of cadaver dissections. Therefore, the skeletal and soft tissue structures of the human head and neck may have significant variations from the accepted anatomic norms. The anatomy of the trigeminal nerve, as described in standard texts, is complex, but not necessarily accurate for a given patient. Perhaps variation in the position of the nerve or associated musculoskeletal structures contributes to the incidence of nerve injuries despite correct surgical techniques. In the largest known cadaver study, Behnia and associates looked at the anatomy of the LN in 669 nerves from 430 fresh cadavers. Measurements on each cadaver were made with a micrometer caliper to determine the horizontal and vertical position of the LN in the lower third molar region. In 94 cases (14.0%), the nerve was above the lingual crest, and in 1 case (0.15%), the nerve was in the retromolar pad region. In the remaining 574 cases (85.8%), the mean horizontal and vertical distances of the nerve to the lingual plate and lingual crest were 2.1 ± 1.1 mm and 3.0 ± 0.4 mm (range, 1.7 to 4.0 mm), respectively. In 149 cases (22.3%), the nerve was in direct contact with the lingual plate of the alveolar process. This study confirms the variable anatomy of the trigeminal nerve in the maxillofacial region.
Similarly, the position of the IAN has great variability. Although the nerve is known to traverse along an interosseous path from the mandibular foramen on the medial mandibular ramus to the mental foramen on the lateral mandibular body, its position within the mandible can have significant interosseous variation superoinferiorly or mediolaterally (or both) and in its relationship to the mandibular molar and premolar teeth ( Fig. 29-4 ). In addition, the position of the nerve changes relative to the alveolar crest if the edentulous mandible undergoes resorption with age.
Diagnostic Studies
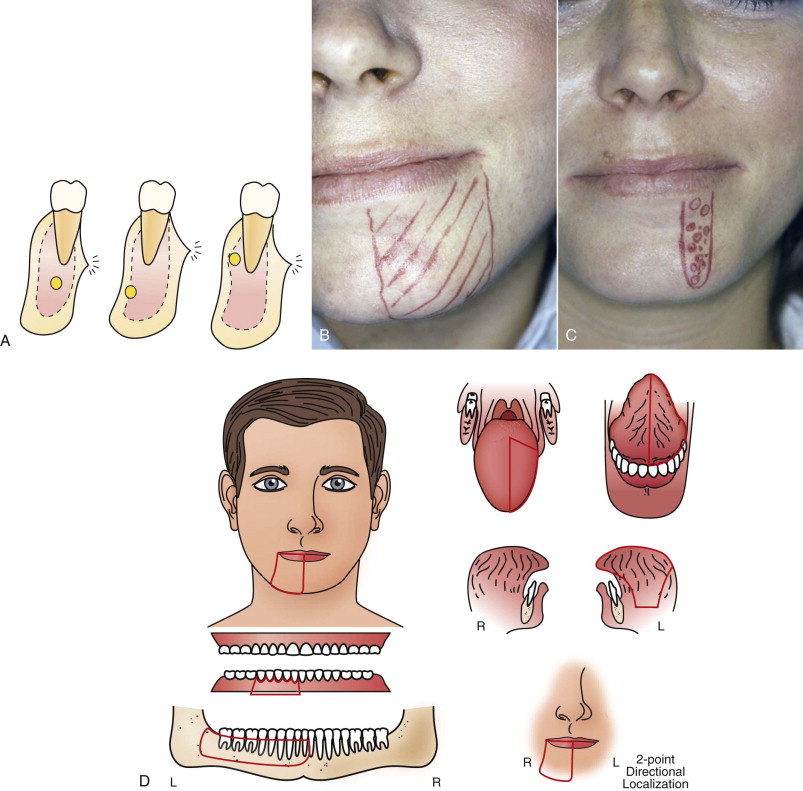
Because neurosensory function cannot be assessed directly, indirect clinical measures of sensation (e.g., temperature discernment, vibration, pinprick, light touch, two-point discrimination) are evaluated for representation of neurosensory function; there have been variable methods of determining these measures.
A modified MRC scale, originally developed for the upper extremities to grade and monitor brachial plexus injuries, has been adapted to assess the functional sensory recovery of trigeminal nerve injuries and make comparisons between studies possible (see Table 29-1 ). The MRC scale provides a global assessment of neurosensory function by using a combination of measures. The scale ranges from a score of S0 (no improvement) to S4 (complete recovery). For peripheral nerve injuries, a score of S3 or higher has been defined as “useful sensory function” (USF). The advantages afforded by this scoring system are to (1) provide objective criteria for classification of results; (2) promote and develop common and accepted use of the scale in all disciplines in which peripheral nerve surgery is performed (i.e., hand surgery, plastic and reconstructive surgery, neurosurgery, oral and maxillofacial surgery); and (3) enable comparison of data in various studies in the literature, even when the scale was not used originally by the study authors.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


