Introduction
The aim of this study was to investigate whether photographic images are accurate and reliable to assess Little’s irregularity index (LII) compared with study model measurements.
Methods
Ten plastic models with different levels of incisor irregularity were photographed occlusally at 9 angulations ranging from 70° to 110° on 3 occasions. Clinical occlusal photographs and study models of 72 dental arches were selected from 16 patients participating in a study of incisor alignment. LII was measured from the models by using digital calipers and from the digital images with ImageJ software (Rasband WS, National Institutes of Health, Bethesda, Md; http://rsb.info.nih.gov/ij/ , 1997-2006).
Results
Photographs of the plastic models and the direct measurements of the models showed high levels of reliability and repeatability (intraclass correlation coefficients [ICC], 0.999 and 0.983). Validity was shown by the limits of agreement between the plastic models and the photographs at 90°, 70°, and 110°, which were –0.2621 to 0.9547, –0.1961 to 1.3205, and –0.7399 to 2.0405, respectively, and the ICC were 0.993, 0.983, and 0.969, respectively. The intraexaminer and interexaminer ICC for the clinical photographs were 0.997 and 0.987, and intraexaminer agreement for the study models was 0.995. The limits of agreement and the ICC between the photographic method and study model method were –1.9656 to 1.2861 and 0.986.
Conclusions
LII can be measured from photographs with good reliability and repeatability. The limits of agreement indicate that, for the mean value of a group of subjects, the photographic method is valid, but care is needed in interpreting an individual measurement.
Little’s irregularity index (LII) was developed and published by Robert Little in 1975, and it is widely used for assessing irregularity, crowding, relapse, and alignment of teeth. The LII measures the horizontal linear distance between anatomic contacts of the incisors in the labiolingual direction parallel to the occlusal plane, ignoring vertical displacement, starting from and ending at the mesial anatomic contacts of the canines. The sum of the 5 measurements is the LII score. The higher the index value, the more severe the labiolingual displacement of the teeth. Little developed his index on the mandibular incisors to look at relapse; however, researchers have used it to evaluate incisor irregularity in both arches in a range of settings. Most studies with the LII have recorded the score from study models by using calipers.
Other methods that have been used to measure the LII can be divided into 2-dimensional and 3-dimensional (3D) methods. Two-dimensional methods include occlusal scanning of study models with a flatbed scanner, photocopies of the occlusal surfaces of the maxillary or mandibular study models followed by either direct measurement or digitizing the photocopied images. These methods have the advantage that, as described by Little, vertical displacement is not considered. Three-dimensional methods include the reflex metrograph, reflex microscope, and use of a 3D digitizer. These 3D methods are prone to errors because the distance between the contact points is recorded in both the horizontal and vertical planes and so might not be a valid representation of the LII. All of these methods require the availability of study models, produced from dental impressions—a time-consuming and sometimes unpleasant procedure for the patient.
Intraoral photographs have previously been used to measure tooth movement and rotations during active treatment, but the study did not report the repeatability or accuracy of this method. Photographs provide a 2-dimensional image of a 3D object, the same as photocopying or scanning with a flatbed scanner. Photocopying and scanning with a flatbed scanner have good reliability and validity compared with measurements from study models. However, photographs can introduce errors of projection, since, unlike a scan or photocopy that is recorded perpendicular to the occlusal plane, the angulation at which the photograph was taken might vary so that a vertical component of the contact point displacement can be introduced to the measurement.
In this study, therefore, we aimed to (1) evaluate the repeatability and reliability of measuring the LII from photographic images of study models compared with the repeatability and reliability of measuring the study models directly with digital calipers; (2) determine the validity of this photographic method by comparing the agreement of the measurements from these photographs with those from the study models; (3) assess the effect of the photographic projection on the measurement and its reliability and validity; and (4) assess the reliability and validity of clinical photographs to measure the LII.
Material and methods
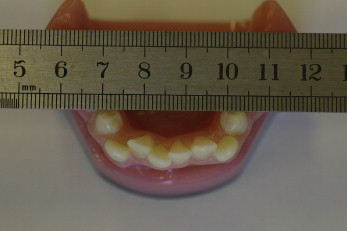
Ten plastic models with different levels of incisor irregularity were photographed occlusally on 3 occasions, 1 day apart. On each occasion, photographs were taken of each model at 9 angulations from 70° to 110° at 5° intervals, giving 270 images. The camera was mounted on a tripod and reset between each photograph by using the built-in protractor to control its angulation ( Fig 1 ). The first photograph was taken parallel to the long axis of the incisor teeth (perpendicular position, 90°),considered the ideal projection. This ideal projection should show the labial and lingual surfaces of the anterior teeth. Each of the 10 plastic models was photographed with a metal ruler crossing the dental arch, contacting the tips of the premolars to allow for calibration of the image ( Fig 2 ).
The models and photographs were randomly allocated codes and then measured twice by using digital calipers for the models and ImageJ software (Rasband WS, National Institutes of Health, Bethesda, Md; http://rsb.info.nih.gov/ij/ , 1997-2006) for the photographs, with a 2-week interval between measurements. For the photographs, the image magnification was calibrated by using 2 points 40 mm apart on the ruler; then the anatomic contact points were identified on the screen, and the contact point displacement was recorded by the program to allow calculation of the LII score. The observer (N.A.) was allowed to use the image zoom tools in the software when this aided in point identification.
Clinical occlusal photographs and matched study models of 96 dental arches were available from 16 patients participating in a study of incisor alignment. This study was approved by the Salford and Trafford Research Ethics Committee of UK National Research Ethics Service, including the taking of photographs for this investigation.
The inclusion criteria for the sample were patients with maxillary and mandibular fixed appliances, minimum pretreatment LII scores equal to or more than 5 mm in either arch at the start of treatment, and all 6 maxillary and mandibular anterior teeth included in the appliance.
We first developed a method of obtaining suitable photographic images for this study. Because of distortion and dimensional changes of objects in the photographs, a customized rigid reference scale was needed for calibration. A disposable millimeter ruler was developed by printing the scale on acetate and bonding this to a clear polypropylene sheet to give greater rigidity. The millimeter ruler was placed with wax to contact the cusp tips of the premolars to be in the occlusal plane, crossing the dental arch horizontally.
To standardize the photographic projection of the images, the operators had training and were given a clinical guide. The occlusal photographs were taken, as far as possible, parallel to the long axis of the incisor teeth, and had to show the labial and lingual surfaces of the anterior teeth and the plastic ruler.
All photographs were taken with a digital camera (S3, Fuji Film, Tokyo, Japan), a 105-mm macro lens (Nikon Corporation, Tokyo, Japan), and a ring flash (Speedlight, Nikon). Alginate impressions were recorded by using BluePrint Cremix (Dentsply, Detrey, Germany). Records were obtained every 4 to 6 weeks at the first 3 routine visits. The records were taken with the brackets in place but the archwire removed.
The models were randomly allocated codes, measured with digital calipers, and the LII scores recorded. Two weeks later, the codes were reallocated and the models remeasured. A second observer then scored all the study models. To record the LII from the digital images, each file name was changed to a random code to ensure that the observer was blinded to the treatment visit. The image was then imported into the ImageJ software and calibrated, and the data were recorded as discussed in the in-vitro study above. A second observer then recorded the LII scores for all digital images.
Assuming a standard deviation of differences in the LII of 0.330, a sample size calculation showed that a 0.050 two-sided Fisher z-score of the null hypothesis (that the correlation coefficient would be 0.990, with no significant difference between the 2 methods, indicating near perfect agreement) would have 90% power to detect a correlation coefficient value of 0.970 or less with a study sample size of 38. We therefore aimed to obtain a minimum sample of 38 matched photographic and study cast records.
Statistical analysis
For the statistical analyses in this study, we used interclass correlation coefficient (ICC), reliability coefficients (Cronbach’s alpha), and mean differences to assess the reliability of the repeated measurements. When comparing the photographic measurements with the study model measurements to determine the validity of the photographic measurements, we also used limits of agreement and plotted Bland-Altman graphs. 28 These graphs plot the mean difference against the mean value and are used when examining 2 methods of measuring the same variable, since they give more information than the simple correlation graphs in this situation. If the differences are normally distributed, 95% of the differences will lie between the limits of agreement. If the limits of agreement are judged to be narrow or can be regarded as unimportant clinically, we can be sure that the new method can be used interchangeably with the old method. 28 This judgment can be based on the amount of variation in the accepted or gold-standard method (in this case, study model measurement) and on current clinical opinion.
For comparison between the plastic study model measurements and the photographs of the study models, we considered whether to aggregate the data from the separate recordings. Although this might give a truer value for the actual measurement and is a common practice in research studies, it might also reduce the error that we would detect and would be present if only 1 measurement was taken, as could occur in a clinical situation. To cover both possible scenarios—research and clinical—we therefore present the data from the aggregated measurements and also the first measurement. To give a more valid assessment of the mean difference between the methods, the values for the difference for each case were converted to positive values by using a root mean square (rms) method, and then mean differences were calculated. This ensures that errors on either side, one with a positive value and one with a negative value, do not cancel each other but, instead, summate . The unconverted data was used for the Bland-Altman plots, and this shows the direction and magnitude of the differences between the photographic and study model measurements.
| ICC | Reliability coefficient |
Mean difference (SD) | |
|---|---|---|---|
| Photo 70° | 0.972 | 0.984 | 0.041 (0.857) |
| Photo 75° | 0.994 | 0.997 | 0.085 (0.387) |
| Photo 80° | 0.969 | 0.986 | −0.334 (0.816) |
| Photo 85° | 0.978 | 0.991 | −0.419 (0.696) |
| Photo 90° | 0.996 | 0.998 | 0.065 (0.341) |
| Photo 95° | 0.992 | 0.995 | 0.011 (0.521) |
| Photo 100° | 0.990 | 0.994 | −0.056 (0.583) |
| Photo 105° | 0.983 | 0.993 | 0.353 (0.647) |
| Photo 110° | 0.970 | 0.984 | 0.265 (0.939) |
| ICC | Reliability coefficient | Mean difference (SD) | |
|---|---|---|---|
| Study model | 0.983 | 0.991 | 0.189 (0.693) |
| Photo 70° | 0.967 | 0.982 | 0.173 (0.281) |
| Photo 75° | 0.994 | 0.997 | 0.109 (0.127) |
| Photo 80° | 0.994 | 0.998 | −0.268 (0.087) |
| Photo 85° | 0.997 | 0.999 | −0.12 (0.091) |
| Photo 90° | 0.999 | 1.000 | −0.061 (0.055) |
| Photo 95° | 0.997 | 0.999 | −0.053 (0.092) |
| Photo 100° | 0.998 | 0.999 | −0.01 (0.077) |
| Photo 105° | 0.997 | 0.999 | −0.031 (0.092) |
| Photo 110° | 0.992 | 0.996 | 0.113 (0.150) |
| ICC | Reliability coefficient |
Rms mean difference (SD) | Mean difference (SD) | Limit of agreement (mean difference ± 1.96 SD) (mm) |
||
|---|---|---|---|---|---|---|
| Study model photo 70° | 0.983 | 0.990 | 0.5622 (0.3792) | −0.0712 (0.6994) | −0.1961 | 1.3205 |
| Study model photo 75° | 0.965 | 0.981 | 0.8158 (0.5264) | −0.1945 (0.9872) | −0.2369 | 1.8685 |
| Study model photo 80° | 0.982 | 0.990 | 0.5473 (0.4048) | −0.1466 (0.6876) | −0.2623 | 1.3569 |
| Study model photo 85° | 0.992 | 0.996 | 0.3767 (0.2512) | −0.0086 (0.4697) | −0.1257 | 0.8791 |
| Study model photo 90° | 0.993 | 0.996 | 0.3463 (0.3042) | 0.0000 (0.4752) | −0.2621 | 0.9547 |
| Study model photo 95° | 0.998 | 0.999 | 0.1722 (0.1644) | 0.0088 (0.2447) | −0.1566 | 0.501 |
| Study model photo 100° | 0.992 | 0.997 | 0.3532 (0.3566) | 0.2698 (0.4299) | −0.36 | 1.0664 |
| Study model photo 105° | 0.979 | 0.993 | 0.5144 (0.5819) | 0.4951 (0.6012) | −0.6483 | 1.6793 |
| Study model photo 110° | 0.969 | 0.984 | 0.6503 (0.6951) | 0.2513 (0.9396) | −0.7399 | 2.0405 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


