• Figure 4-1 Periodontal laser treatment for a tight lingual frenum. The patient was referred by a speech pathologist for surgical correction. A, Presurgical view of the frenum. B, Beginning incision for release of lingual frenum with CO2 laser. Note excellent hemostasis and conservative incision. C, Immediate postoperative view of completed lingual frenectomy. D, Lingual frenectomy site 3 days after surgery. Note excellent and rapid healing. © Dr. Robert A. Convissar.
• Figure 4-2 Use of a laser to gain adequate intraoral surgical access in a medically compromised patient on anticoagulation therapy. A, Presurgical view of a buried orthodontic chain. B, Immediate postoperative view after exposure of the orthodontic chain accomplished by means of a laser. The laser tip was moved in an apical direction while tension was placed on the exposed chain. With minimal hemorrhage, clear access to the wound area has been obtained. The chain can now be successfully incorporated into the orthodontic appliance.
Although the use of lasers in initial periodontal therapy has significant advantages, it must be emphasized that laser treatment is an adjunct to standard therapy rather than a replacement therapy. For example, with standard nonsurgical scaling and root planing, the goals include reduction of the bacterial biofilm, removal of necrotic cementum and subgingival calculus, and deepithelialization of the sulcus. Scaling and root planing should result in decreased inflammation, decreased pocket depth, and attachment gain through a long junctional epithelial attachment.10 Lasers do not remove necrotic cementum or subgingival calculus but do aid in reduction of the biofilm and deepithelialize tissue more quickly and easily than with conventional techniques. Rossmann et al.11 emphasized that CO2 lasers can be used to delay the apical downgrowth of epithelium, and that this technique is less technically demanding and more time-efficient than other techniques.
The Nd:YAG and diode lasers are of limited use in hard tissue root therapy for root detoxification because they are primarily soft tissue lasers. By contrast, erbium lasers demonstrate capability for root debridement by their effect on calculus and necrotic cementum, with reduced endotoxins.12,13 Also, evidence indicates that these effects can increase the attachment level gain over that achieved with scaling and root planing.14,15 However, systematic reviews (especially evidence-based) demonstrate minimal differences in nonsurgical periodontal end points between laser and conventional periodontal therapy. Evidence shows that soft tissue laser curettage does not contribute to additional gains in attachment level beyond those obtained with meticulous periodontal root planing in chronic adult periodontitis. Therefore soft tissue lasers such as the Nd:YAG and diode, with their ability to deepithelialize the gingival sulcus and some antibacterial properties, may have limited application for nonsurgical periodontal therapy. The erbium laser, in addition to soft tissue use, may be the device required for calculus removal and hard tissue detoxification, creating a biocompatible surface for attachment of connective or epithelial tissue.16 Using the CO2 wavelength, Crespi et al.17 found that they could increase the quality and quantity of fibroblasts attaching to the root surface.
As an adjunct to periodontal debridement, photodynamic therapy may have potential. In one such approach, with use of either a “cold” (low-level) laser or a conventional dental laser with wavelengths absorbed by pigment (e.g., diode, Nd:YAG), methylene blue dye is placed in the sulcus as a subgingival irrigant. These laser wavelengths are attracted to and interact with the dye, disrupting the bacterial cell membranes. The light energy activates the dye, interacts with intracellular oxygen, and destroys the bacteria by lipid peroxidation and membrane damage. Chapters 3 and 15 discuss the use of lasers for initial (nonsurgical) periodontal therapy.
Gingivectomy
Gingivectomy is a time-honored procedure for removal of gingiva. The indications range from access to esthetics. The gingivectomy can be used when suprabony pockets are present and access to osseous structures is not necessarily important. The procedure assists in decreasing gingival tissue in cases of enlargement and in altering fibrotic gingiva (Figure 4-3). However, gingivectomy is contraindicated when (1) access to osseous structure is critical or (2) gingival attachment is inadequate (minimal) or absent.
Clinical observation demonstrates that resecting gingiva with a laser enhances access because of increased visualization resulting from sealing of capillaries and lymphatics during laser irradiation. In the early stage of tissue healing with use of blades, inflammation is noted, along with collagen production and epithelialization, and the wound has a high tensile strength.
The laser wound generally demonstrates delayed epithelialization, collagen production, and inflammation, with a lower tensile strength. In later phases of healing, however, the process accelerates, with collagen production and epithelialization. Myofibroblasts are present in fewer numbers during healing of a laser-resected wound site, which leads to less wound contraction and less scar formation18 (Figure 4-4).
As discussed earlier, no clear conclusion has emerged regarding healing rates for laser-induced wounds versus conventional scalpel wounds. However, Nd:YAG, CO2, erbium-doped YAG (Er:YAG), and diode lasers demonstrate wound healing that is either comparable with that achieved after use of conventional scalpel blades or somewhat accelerated.19 White et al.20 compared several laser technologies using histologic analysis and determined that wound healing is influenced by certain instrument settings—W, Hz, pulse duration, and time of exposure. It could be concluded, therefore, that wound healing after a flap or a gingivectomy procedure using laser therapy depends as much on device settings as on the actual laser wavelength used. Also, training often is as important as or sometimes more important than laser wavelength.
• Figure 4-3 Excision versus incision. The excisional gingivectomy incision is created on an external bevel (small arrow). If the bevel cannot be created owing to difficulties with access, the incision can be blended into the apical gingiva using the laser tip. The internal incision (large arrow) also can be created for purposes of the flap procedure. (Modified from Rose LF, Mealey BL: Periodontics: medicine, surgery, and implants, St Louis, 2004, Mosby.)
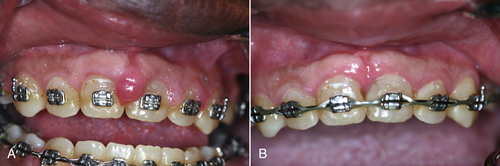
• Figure 4-4 Laser gingivectomy for treatment of gingival hyperplasia. A, Presurgical view. B, Ten days after the laser procedure. The cause of the hyperplasia was lack of oral hygiene compliance exacerbated by loading from orthodontic appliances.
An alternative to laser therapy, electrosurgery (especially monopolar devices), does not have a defined target tissue as with laser technology. The primary mode of tissue interaction with electrosurgical instruments is by heat ablation. The zone of necrosis after electrosurgery can be 500 to 1500 μm. Diode and Nd:YAG lasers can generate heat in tissue at a depth of up to 500 μm, whereas erbium and CO2 lasers, because of high water absorption, penetrate from 5 to 40 μm. Bipolar electrosurgery units constitute an improvement over monopolar units in that bipolar units generate less lateral heat and can be used in a wet environment.21
Treating noninflamed, fibrotic gingiva with diode and Nd:YAG lasers requires different power settings than for hyperemic or vascular tissue.22 Lasers are attracted by specific chromophores, so less power is needed to incise tissue if abundant chromophore is present in the tissue. When the gingiva is hyperemic and inflamed, less power is needed because of the high amount of chromophore (hemoglobin) in the tissue. More power is needed to incise fibrotic tissue with less chromophore (hemoglobin) present. The same holds true for gingivectomies in patients with higher melanin content of tissues. Melanin is one of the chromophores of Nd:YAG and diode lasers. Tissue that is heavily pigmented with melanin requires much less power than gingiva that is very light, coral pink when theses wavelengths are used.
In performing surgery with CO2 and erbium lasers, melanin and hemoglobin content is less important. Erbium and CO2 laser wavelengths are absorbed primarily by water, so both of these lasers will require less power for incising hyperemic tissue than for cutting through fibrotic tissue. An erbium laser can be used for a gingivectomy, but hemostasis can be problematic with this wavelength. Some clinicians may follow an erbium laser procedure with use of a diode, Nd:YAG, or CO2 laser to achieve coagulation if hemorrhage is a problem. Others use erbium laser settings that create a so-called laser bandage (settings of low wattage, no water, and some air, with fewer pulses per second). In the past, this laser bandage was referred to as a “char layer” or an “eschar.” Although older lasers routinely created a char layer because of their high fluences, newer laser units rarely char tissue. Use of a char layer will depend on the clinician’s preference, because the findings reported in the literature are equivocal.
The clinician must appreciate both the emission spectrum of the laser and the absorption spectrum of the tissue. What wavelength is being emitted? What is the primary chromophore of the tissue? How does tissue biotype affect the laser parameters? All of the variables of laser use—power, spot size, pulses per second, and hand speed—must be taken into account, along with wavelength and tissue biotype (see Chapter 2). It is a mistake to consider that increasing power alone may result in quicker cutting through the target tissue. Lasers generate heat that may result in tissue necrosis from lateral thermal damage. Therefore settings are critical in performing laser therapy. Again, it must be emphasized that training should be one of the primary considerations in evaluation of the different laser manufacturers before purchase.
Initial incisions for gingivectomies are similar to that of using a blade with an external bevel approach. The distance of the incision from the coronal gingival margin is based on pocket depth and amount of existing attached gingiva. A gingival chamfer (beveled edge) is achieved, rather than a direct right angle into the gingiva. Thus the initial cut is made slightly apically to the pocket depth measurement. A slow, unidirectional hand motion is used, moving the tip at an external bevel toward the tooth structure. Caution is necessary in approaching the tooth, especially near root structure, because of the possible laser–hard tissue interaction, which could result in tissue damage. Decreasing the power will prevent such injury; if the power is decreased, however, multiple passes over the incision line may be necessary to complete the cut. Delivering laser energy repeatedly over tissue that has already been treated may result in a greater extent of lateral thermal damage.
Some clinicians use a reflective barrier in the sulcus to prevent the wavelength from interacting with the root. Placing a thin, sterile #7 wax spatula or a small periosteal elevator, or even a piece of metal or mylar matrix band, in the sulcus between the tooth and the soft tissue will prevent any laser energy from damaging the hard tissue; the metal or mylar will reflect the laser energy away from the tooth. Once the gingiva has been excised, power-driven ultrasonic scaling is used to debride the root surface.
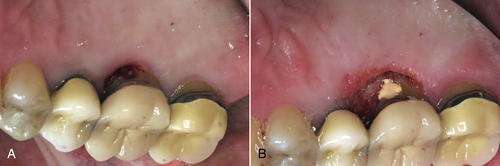
• Figure 4-5 Laser gingivectomy performed to gain access for restoration prognosis: preoperative (A) and immediate postoperative (B) views. The patient presented with a possible carious lesion detected subgingivally with an explorer on examination. To determine the extent of the lesion, gingivectomy was performed with a diode laser. Note excellent hemostasis.
Because of the sculpting ability of lasers, gingivoplasty can now be performed to smooth the gingival margins for a parabolic appearance. With diode, CO2, and Nd:YAG lasers, hemostasis is achieved during the procedure. Erbium lasers create hemostasis after the procedure by altering laser parameters to seal blood vessels. Again, some clinicians incorporate the erbium laser bandage as the final procedure. Whether or not to place a dressing is a matter of clinician preference (Figure 4-5).
Frenectomy
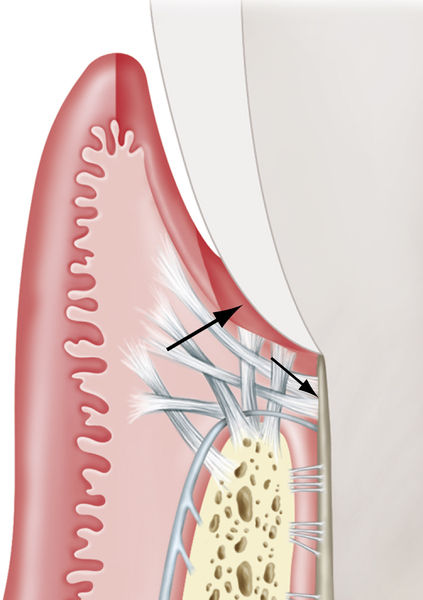
Use of the frenectomy procedure in periodontics (unlike in orthodontic or pediatric applications) is limited because of the minimal increase in attached gingiva observed after postfrenectomy wound healing (Figure 4-6). Alveolar mucosa is characterized by a red color and a smooth surface and generally is loose and mobile, whereas attached gingiva is keratinized, pink, stippled, and firm, with no mobility (Figure 4-7).
Gingival surgery in the form of soft tissue augmentation is necessary under the following conditions:
• Marginal gingiva is inflamed.
• Bleeding or exudate is present at the sulcus/pocket.
• Obvious recession is present.
• Pulling of marginal tissue occurs with retraction of the lip.
• As determined by direct measurement, the sulcular depth in millimeters is subtracted from the keratinized zone of gingiva. At least 2 mm of “keratinized attached gingiva” should be present.
Frenectomy procedures with a laser are predictably successful so long as the following steps are incorporated:
1. Creation of a periosteal fenestration at the base of the frenectomy to prevent reattachment of fibers
2. Removal of all impeding muscle fibers
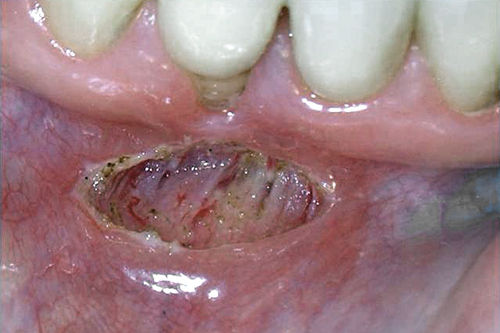
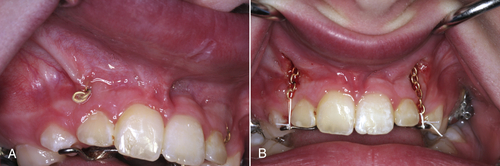
• Figure 4-6 Immediate postoperative view of laser frenectomy site in a patient in whom the frenectomy was performed without consideration for the extent of gingival attachment, which was minimal to none. A mucogingival graft procedure or vestibuloplasty is indicated to gain an adequate amount of keratinized attached gingiva. Note hemostasis.
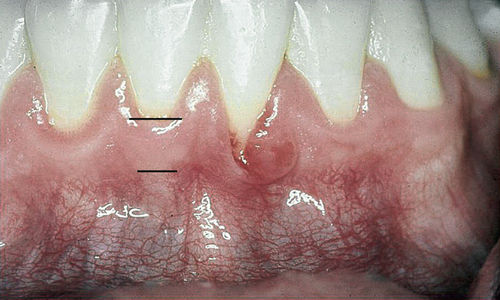
• Figure 4-7 Comparison of alveolar mucosa and gingiva. Parameters of color, surface, and mobility are used in differentiating mucosa from gingiva. Mandibular left lateral incisor has a cleft that borders on alveolar mucosa. The level of inflammation makes discerning the level of attached gingiva difficult; this determination is best delayed until after health is achieved.
All laser wavelengths can be used to perform a frenectomy successfully; however, depth of penetration for diode and Nd:YAG lasers is much higher (500 μm) than for erbium or CO2 lasers (5 to 40 μm), so settings must be monitored closely to prevent thermal damage to the underlying periosteum and bone.23 In some patients, topical anesthesia is sufficient to allow performance of a frenectomy, with excellent precision, and with less discomfort and shorter healing time than with conventional techniques. CO2 laser treatment for frenectomy provides better postoperative patient perceptions of control of pain and improved function than with the scalpel technique.24 Likewise, use of the Nd:YAG laser may result in less postoperative pain and fewer functional complications.25
The technique for a laser frenectomy is similar to that using a blade (Figure 4-8). Local or topical anesthesia is administered. The clinician should first visualize the procedure by forming a mental outline of the incision. This incision begins at the coronal attachment; the laser tip is then moved unidirectionally, with tension achieved by pulling on the lip. With use of the correct parameters (spot size, power, hand speed), one pass of the laser should be sufficient to sever all of the fibers. If multiple passes are necessary, care must be taken to avoid excessive lateral thermal necrosis from reexposure of already-treated tissue. The laser incision is continued to undermine the muscle attachment until the periosteum is reached.
To ensure minimal regrowth and prevent frenum “relapse,” the periosteum should be fenestrated with a hand instrument. All lasers are effective for a frenectomy with settings suggested by the manufacturer. Care must be taken not to char the tissue, with consequent thermal tissue damage. The erbium laser creates a wound that may exhibit some hemorrhage, so sealing the wound with the laser bandage (see Fig. 4-9) approach may be required. No suturing or dressing is necessary (see also Chapters 11 and 12).
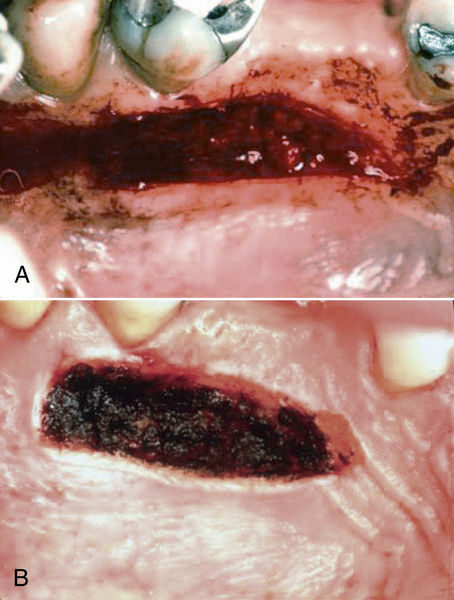
• Figure 4-9 Use of a laser in a mucogingival procedure to “seal” the surgical wound for control of hemorrhage. A, Donor site immediately after harvesting of a graft in conventional fashion with a blade. B, Donor site immediately after creation of a “laser bandage” for local hemostasis. (Courtesy Dr. Stuart Coleton.)
Mucogingival Surgery
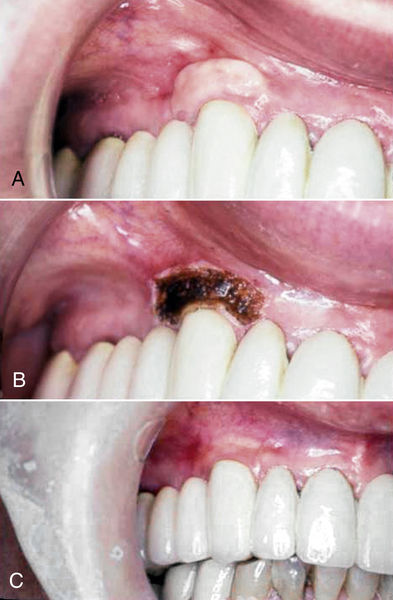
Lasers can be used in mucogingival procedures for a variety of therapies. Donor material can be acquired from the palate or other keratinized areas in the oral cavity with laser therapy. Using a laser to “seal” the wound when donor material is taken from these areas using blades can reduce hemorrhage significantly (Figure 4-9).
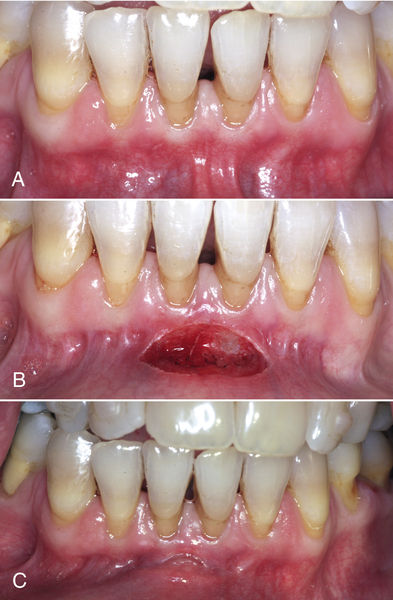
In some patients, the resulting recipient area may be overcontoured at several weeks postoperatively if an overly thick donor graft was used. Any soft tissue laser can be used to recontour the site, with a positive esthetic result (Figure 4-10).
In other patients, rather than performing a graft procedure, the zone of attached tissue may be increased by a vestibuloplasty. Although the preoperative view in Figure 4-11A shows an insufficient amount of attached gingiva, the underlying problem is that the fibers are very tightly bound together. A vestibuloplasty releases the tightly bound fibers, resulting in a wider band of attached gingiva, without the need for a graft procedure.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses