Figure 5.1 • Absorption spectrum for water. Data was calculated from Hale and Querry (4). Ar (488 nm), Diode (810 nm), Nd:YAG (1,064 nm), Er,Cr:YSGG (2,780 nm), Er:YAG (2,940 nm), and CO2 (10,600 nm) are indicated in the figure.
Figure 5.2 • Er:YAG laser apparatuses. (a) Er:YAG laser VersaWaveTM; HOYA ConBio, CA, USA. Wavelength: 2,940 nm. Output energy range: 30-400 mJ. Pulse repetition rate: 3-50 Hz. Pulse width: 200 µseconds. (b) Er:YAG laser Erwin AdvErlTM; Morita Mfg. Corp., Kyoto, Japan. Wavelenth: 2,940 nm. Output energy range: 30-350 mJ. Pulse repetition rate: 1-25 Hz. Pulse width: 250 µseconds.
(c) KEY Laser 3TM; KaVo, Biberach, Germany. Wavelength: 2,940 nm. Output energy range: 10-600 mJ. Pulse repetition rate: 1-25 Hz. Pulse width: 250-500 µseconds.
ADVANTAGES OF LASERS IN PERIODONTICS
Laser irradiation can achieve excellent tissue ablation with a strong bactericidal effect as well as sufficient haemostasis. Also, high accessibility may be an advantageous property of laser treatment. The adjunctive or alternative use of lasers may help to facilitate the periodontal procedure, and have potential to improve the healing process.
Regarding periodontal pocket treatment, lasers are one of the most promising new modalities. Er:YAG laser irradiation has been reported to exhibit bactericidal and detoxification effects without producing a smear layer on the root surface, and the laser-treated root surface provided favorable conditions for the attachment of periodontal tissue. Furthermore, contrary to mechanical treatment with conventional instruments, laser therapy is expected to promote the healing of periodontal tissues through a curettage effect of ablating the inflamed lesions and epithelial lining of the soft tissue wall within the periodontal pockets [8]. Also, part of the laser energy scatters and penetrates during irradiation into the periodontal pockets and, therefore, the attenuated laser at a low energy level might then stimulate the cells of surrounding tissue, like low level laser therapy (LLLT) [8], resulting in a reduction of the inflammatory conditions and in activation of cell proliferation, improving the periodontal tissue attachment and also possibly reducing postoperative pain [9].
I. SOFT TISSUE APPLICATION
Gingival procedures
Due to easy tissue ablation with strong haemostasis and bacteria killing effects, lasers are frequently employed for soft tissue procedures. Gingivectomy, gingivoplasty and frenectomy are the most popular procedures using lasers [3] (Figure 5.3).
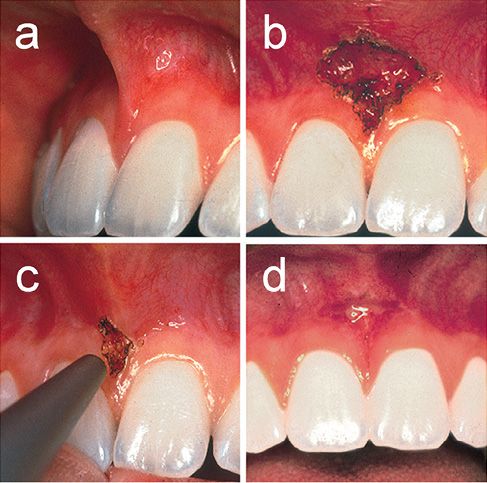
Figure 5.3 • Frenectomy using a CO2 laser. (a) Before treatment. Sixteen year old female. (b) High attachment of frenum was easily ablated and resected with the continuous CO2 laser at 4 W under local anesthesia in non-contact mode. (c) The laser treated surface shows carbonization but lacks bleeding. (d) Wound healing was uneventful and favorable at 1 week.
Lasers can easily incise, cut, ablate and reshape the soft tissue with no or minimal bleeding and less pain. Laser surgery occasionally requires no local anesthetic or only a topical anesthetic [10]. Lasers of the deeply-penetrating type, such as Nd:YAG and diode lasers, show a thicker coagulation layer than lasers of the superficially-absorbed type, such as CO2 and Er:YAG lasers. Little wound contraction and minimal scarring are other advantages of laser surgery. Lasers can be used for the treatment of gingival hyperplasia, frenectomy, crown lengthening and the removal of epulis or benign tumors.
With CO2 lasers, excessive soft tissues are easily vaporized. CO2 laser treatment is an extremely fast procedure and generally causes patients no postoperative complications. Gingival hyperplasia is a typical indication for CO2 laser treatment due to its speedy ablation and strong hemostasis [11]. The CO2 laser is also effective in performing gingivoplasty for small tissue aberrations seen after periodontal surgery.
The Nd:YAG and diode lasers can also be used to cut and reshape soft tissues [10,12]. The lasers are brought into contact with the tissue to be operated on, and the tissue is vaporized by moving the tip in a sweeping motion. After lasing, the area is completely coagulated, leaving a surface that will heal uneventfully with no postoperative pain for the patient. The technique used with an Nd:YAG or diode laser is similar to removing the tissue with electrosurgery.
AESTHETIC gingival procedures
Melanin hyperpigmentation is a cause of “black gums”, and the depigmentation is usually performed for aesthetic reasons.
The laser is one of the methods used to treat the condition. The CO2, diode and Nd:YAG lasers can perform melanin depigmentation effectively [13,14]. However, these lasers are occasionally dangerous to the thin gingiva due to their strong thermal and/or deeply-penetrating effects, resulting in gingival ulcer and recession [15].
Recently, the Er:YAG laser has been effectively applied for minor periodontal soft tissue management without major thermal side-effects and the delay of wound healing [6,8,16].
The width of the thermally affected layer in gingival connective tissue is only 5-20 µm after Er:YAG laser melanin removal in dogs. Due to its extremely low thermal effect, this laser is useful and safe for delicate esthetic soft tissue procedures, such as melanin hyperpigmentation [17] (Figure 5.4).
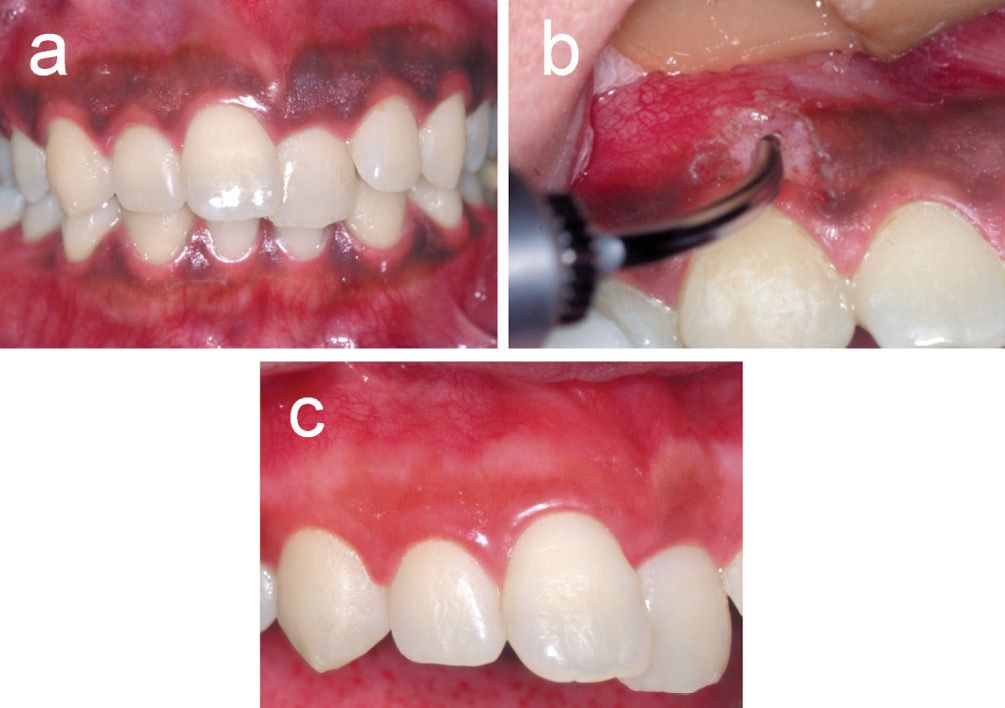
Figure 5.4 • Removal of gingival melanin hyperpigmentation using an Er:YAG laser. (a) Gingival tissue with a band of severe pigmentation before surgery. 27 year old female. (b) Er:YAG laser irradiation was performed at an energy output of 52 mJ/pulse (18.4 J/cm2/pulse) and 30 Hz with water spray in contact mode using a sweeping motion under local anesthesia for the removal of melanin hyperpigmentation. The gingival epithelium containing pigmentation was easily and effectively removed by Er:YAG laser irradiation. No major thermal damage, such as carbonization, was observed. (c) At 3 months after the procedure, favorable wound healing was observed at the treated site without any gingival tissue defect or recession. The gingival colour recovered a natural aesthetic appearance.
The application of the Er:YAG laser, sometimes in combination with a surgical microscope, renders the procedure more precise. The application of a surgical microscope seems to be important during irradiation for the detection of slight remaining pigmentation, and during careful irradiation to the delicate area of the gingival margin and papilla. It becomes possible to deal with the soft tissues delicately, thus yielding satisfactory results compared to those obtained by conventional methods [17].
Furthermore, the Er:YAG laser can be utilized for the removal of abnormal gingival discoloration, namely metal tattoos [6,17]. It is suspected that dental treatment procedures or restorative materials cause this kind of discoloration.
A recent report indicated that this kind of discolored gingiva contained silver sulfate, tin sulfate and pieces of iron, as observed by electron probe microscopy.
Effective removal of the discolored gingiva, together with metal fragments, is possible using an Er:YAG laser, with no postoperative pain or gingival recession (Figure 5.5). Especially, laser microsurgery facilitates this procedure.
III. NON-SURGICAL PERIODONTAL POCKET THERAPY – BASIC STUDIES
Root debridement
Periodontal treatment aims to recover the biological compatibility of periodontally-diseased root surfaces for the subsequent attachment of periodontal tissues to the treated root surface.
In periodontal pockets, the root surfaces are contaminated with an accumulation of plaque and calculus, as well as infiltration of bacterial endotoxins into the cementum.
Usually, debridement of the diseased root surface is performed by mechanical scaling and root planing, mainly using manual or power-driven instruments. However, conventional mechanical debridement using curets is still technically demanding and time consuming, and power scalers sometimes cause uncomfortable stress, such as noise and vibration, in the patients.
Complete removal of bacterial deposits and their toxins from the root surface within the periodontal pockets is not necessarily achieved with conventional mechanical therapy. In addition, access to areas such as furcations, concavities, grooves and distal sites of molars is limited.
Recently, the advantageous effects of lasers, such as ablation, bactericidal and detoxification effects, have been considered to be useful for periodontal treatment, and the application of lasers has been suggested as an adjunctive or alternative tool to the conventional periodontal mechanical therapy [8].
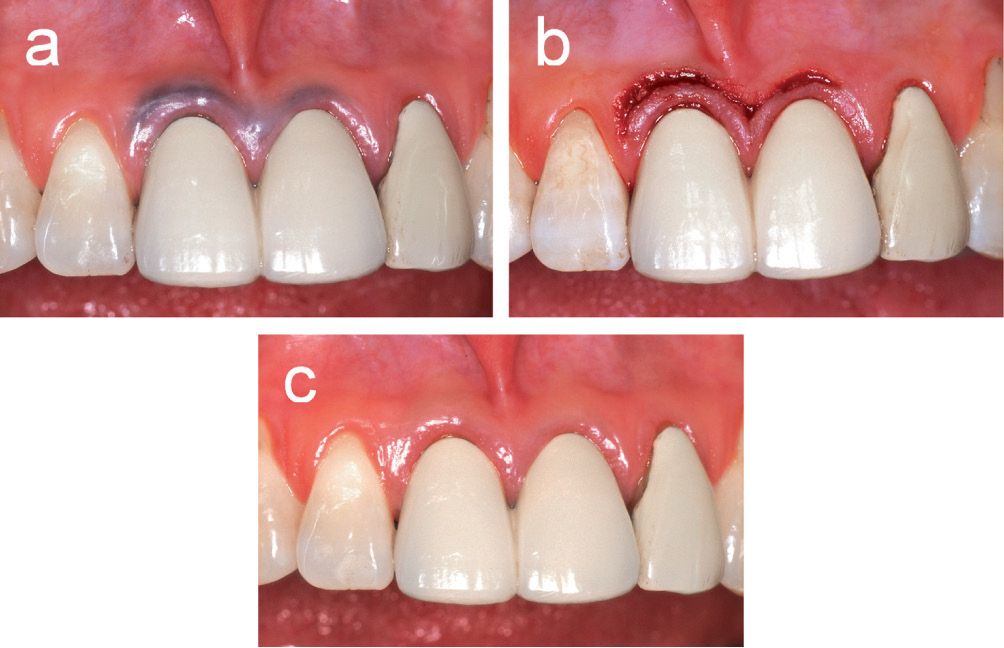
Figure 5.5 • Treatment of abnormal gingival discolouration (metal tattoo) using an Er:YAG laser. (a) A fifty-four year old female presented with abnormal gingival discolouration at the marginal area of central incisors before treatment. (b) Immediately after irradiation. Laser irradiation was performed at an energy output of 40 mJ/pulse (14.6 J/cm2/pulse) and 30 Hz with water spray under local anesthesia in combination with a surgical microscope at a maginification of 20-30 times. The discoloured gingival tissue was effectively ablated and the metal debris was safely and precisely removed. The patient did not experience any stress or vibration during irradiation, and did not complain of any postoperative pain. (c) After treatment, the wound healing was favorable without any necrosis of the marginal gingiva. One year after treatment, the colour of gingival tissue improved markedly without unaesthetic exposure of the root surface due to recession and any recurrence of discolouration. (Photographs from Ishikawa I et al. Potential applications of Erbium:YAG Laser in Periodontics. J Periodont Res; 2004; 39:275-285; with permission. Journal of Periodontal Research ©Copyright (2004) Blackwell Munksgaard, Inc.).
Removal of subgingival calculus and thermal generation
The CO2 laser easily ablates dental plaque on the root surface; whereas, the laser causes melting and carbonization on the dental calculus [18]. The Nd:YAG laser is basically ineffective for calculus removal [19]. Although the Nd:YAG laser can ablate calculus when a higher energy is employed, the treated surface with complete evaporation of the calculus has been shown to have some degree of thermal damage to the underlying cementum [20].
Unlike these lasers, the Er:YAG laser is capable of removing subgingival calculus without a major thermal change of the root surface [4, 8, 21] (Figure 5.6).
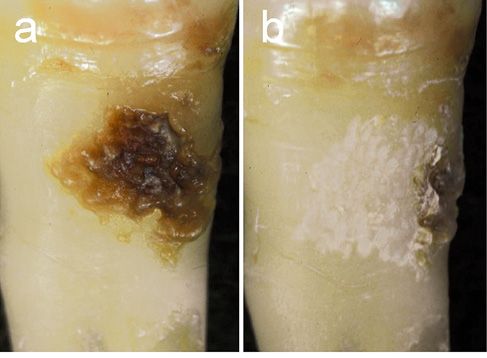
Figure 5.6 • Photographs of the root surfaces before and after Er:YAG laser scaling of the subgingival calculus. Er:YAG laser scaling was performed in vitro at an energy output of 30 mJ/pulse (energy density 10.6 J/cm2/pulse) and 10 Hz under water spray. (a) Extracted tooth with subgingival calculus before laser scaling. (b) After laser scaling. Effective removal of subgingival calculus without any visible thermal damage of the root surface. The lased surface shows a chalky appearance due to the microstructured surface caused by the slight ablation of cementum. Part of the subgingival calculus was intentionally left on the edge of the treated root surface. (Photographs from Takasaki AA et al. Erbium:YAG Laser in Periodontics. Dentistry in Japan; 2006; 42:201-206; with permission. Dentistry in Japan ©Copyright (2006) Japanese Association for Dental Science).
The level of calculus removal by laser scaling is similar to that of ultrasonic scaling, and the depth of cementum ablation has been reported generally 15-30 µm [21]. Furthermore, the Er:YAG laser treatment in vivo might provide selective subgingival calculus removal to a level equivalent to that provided by scaling and root planing (SRP) [22].
However, clinical research reported a lower degree of calculus removal with the Er:YAG laser than with SRP [23].
Regarding thermal generation, lasers of the deeply-penetrating type, such as diode and Nd:YAG lasers, carry the risk of intrapulpal temperature elevations during laser irradiation on the root surface [24]. In the case of the Er:YAG laser, the use of water coolant can effectively prevent thermal generation during laser scaling while not compromising the efficiency of laser scaling [4,21]. A recent study showed that no differences were observable histologically in the pulp tissue at 12 weeks after surgery between the Er:YAG laser treated tooth and conventional mechanical scaling with hand curets [25]. Therefore, it may be presumed that Er:YAG laser subgingival scaling at a low energy level under water irrigation does not produce any major deleterious effect in the pulpal tissue.
Root surface alteration
The CO2 laser readily carbonizes the root cementum [26], and cyan-derived toxic products, such as cyanamide and cyanate ions, have been detected on the carbonized layer by chemical analysis using Fourier transformed infrared (FTIR) spectroscopy [27]. The residual char layer has been demonstrated to inhibit periodontal soft tissue attachment in vivo [28].
Regarding the Nd:YAG laser, irradiation at 1.25-1.50 W (62.5-75 mJ/pulse, 20 Hz) produced surface pitting and crater formation with charring, carbonization, melting and crater production, even when irradiation was performed parallel to the surface [29]. Also, cementum treated with the Nd:YAG laser showed a decrease in the protein/mineral ratio and the potential surface alteration by protein by-products, such as cyanamide and cyanate ions, by FTIR analysis [27,30].
The Nd:YAG laser-treated root surface has been reported to be unfavorable for fibroblast attachment in vitro [31]. However, alterations in the laser-irradiated surface are reversible, and additional root treatment, such as root planing or polishing with air-powder abrasive following Nd:YAG laser irradiation appears necessary in order to render the root surface biocompatible [32].
Several studies have described a characteristic morphological change of the root surface after Er:YAG laser irradiation.
The laser-treated root surface under water coolant has been reported to have a micro-irregular appearance without cracks or thermal side effects, such as melting and microfracture, which are usually observed after CO2 or Nd:YAG laser irradiation [21]. The superficial layer of the root surface ablated by Er:YAG laser irradiation presented a minimal affected layer with characteristic staining [21] and without major compositional or chemically deleterious changes of the root cementum and dentin [26].
Regarding the biocompatibility of the Er:YAG lased root surface, when a suitable energy is selected, the surface structure after Er:YAG laser irradiation offers better conditions for the adherence of fibroblasts in vitro than the root surface after mechanical scaling alone [33,34].
Regarding the diode laser, lasing dry or saline-moistened root specimens resulted in no detectable alterations; however, the blood-coated specimens showed severe damage depending on the irradiation condition [35].
Bactericidal and detoxification effect
It has been proven that conventional methods for the treatment of periodontal disease are not completely effective in eliminating all types of bacteria.
Although systemic and local administration of antibiotics into periodontal pockets is occasionally performed for disinfection, the frequent usage of antibiotics bears the potential risk of producing various resistant microorganisms.
These limitations have led to a shift in emphasis from a purely mechanical approach to the use of a novel technical modality. Now, laser treatment is considered to be a promising adjunctive or alternative procedure.
The defocus mode of the CO2 laser has root conditioning effects, such as smear layer removal, decontamination [36-38] and the preparation of a surface favorable to fibroblast attachment [39].
Although the effect of smear layer removal has also been reported by Nd:YAG laser treatment [40,41], the irradiation parameters used may not be appropriate for clinical use considering the significant rise in the intra-pulpal and root surface temperature.
However, several researchers reported the decontamination effect [42] and the inactivation of the endotoxins in the periodontally-diseased root surface [43] using the Nd:YAG laser. The Er:YAG laser exhibits a high bactericidal effect against periodontopathic bacteria at a low energy level [44,45]. In addition, this laser has the potential to remove toxins diffused with the root cementum, such as the lipopolysaccharide [46].
Also, the bacterial killing effect of argon laser radiation may be effective in the treatment of clinical infections caused by biofilm-associated species, such as Prevotella and Porphyromonas [47,48].
Summary of the in vitro effects of the laser for root debridement
The CO2 laser, when used with high-energy output, especially in continuous wave mode, is not appropriate for calculus removal and root surface debridement due to the major thermal side effects, such as carbonization of the root surface.
However, when used with relatively low energy output in a pulsed and/or defocused mode, this laser may provide root conditioning, detoxification and bactericidal effects on the contaminated root surfaces.
The ability of the Nd:YAG laser to remove calculus is also insufficient, and this laser should be used mainly for the decontamination of gingival tissue.
Meanwhile, the Er:YAG laser is the most promising for root surface debridement, including calculus removal as well as decontamination.
NONSURGICAL PERIODONTAL POCKET THERAPY – CLINICAL STUDIES
Nd:YAG laser
Using a flexible optical fiber, periodontal pocket treatment using the Nd:YAG laser began in the early 1990s. Cobb et al. [49] performed Nd:YAG laser irradiation (1.75-3.0 W : 87.5-150 mJ/pulse and 20 Hz) into periodontal pockets, and showed the ineffective and patchy removal of calculus with root surface alterations similar to those observed in the in vitro studies and a decreased number of bacteria in periodontal pockets after laser treatment. They suggested that laser therapy should be followed by mechanical debridement. Radvar et al. [50] reported that after Nd:YAG laser pocket treatment, the low Nd:YAG laser energy levels employed (0.5 or 0.8 W : 50 or 80 mJ/pulse and 10 Hz) did not cause any heat damage to the root surface, but they failed to improve the clinical and microbiological parameters of periodontal disease as compared to SRP.
Gold and Vilardi [51] performed Nd:YAG laser curettage (1.25 and 1.75 W : 62.5 and 87.5 mJ/pulse and 20 Hz) in vivo
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


