• Figure 7-1 A, Large surgical incision made with an ultraspeed CO2 laser for a sinus lift, closed with continuous, sling-locking sutures. B, Photograph of site 48 h postoperatively. Note normal tissue color and relaxed sutures, with minimal evidence of swelling.
The fiber delivery system of diode and Nd:YAG lasers allows debris to build up on the fiber tip. Consequently, frequent cleaning and cleaving of the tip are necessary.19 Uncovering implants, if the tissue is relatively thin, is an appropriate use for a diode. A full-thickness flap or incision down to the periosteum to place implants is much more difficult with a diode than with a CO2 laser.
In summary, a 980-nm diode laser can be used safely for some implant procedures, but with limitations in the depth of cut, speed of cut, and efficiency of cutting. The major advantages of a diode laser are its small size and relatively low cost.
Neodymium:Yttrium-Aluminum-Garnet Lasers
The Nd:YAG lasers operate at a wavelength of 1064 nm. These lasers are fiberoptic-delivered contact lasers that generate a free-running pulsed beam of energy. This pulsing mechanism is more sophisticated and the potential for heat penetration even greater than with a diode laser. The 1064-nm wavelength is poorly absorbed in water but readily absorbed into tissue pigments such as hemoglobin and melanin. The Nd:YAG laser is effective at producing coagulation and hemostasis but, because of its penetrating depth of up to 4 mm, has the greatest potential for damaging soft and hard tissues as well as implant surfaces.16 The energy is delivered through the carbonized tip of a fiber, as with a diode laser. However, the maximum peak power emitted by the Nd:YAG is much greater than for a diode and therefore could penetrate the carbonized debris at the laser tip.20
The Nd:YAG laser is useful in periodontal therapy and has had positive effects in pocket therapy.21 However, Block et al.22 report that the Nd:YAG laser energy can melt the surface of implants or remove the surface layer from plasma-coated titanium implants. This laser also produces craters and cracks on different surfaces of titanium. Furthermore, Walsh23 and Chu et al.24 found contraindications to the use of Nd:YAG lasers near implants. Although anecdotal reports have described use of this wavelength in periimplant therapy, and one manufacturer is promoting a specific laser-assisted periimplantitis protocol, to date, no studies showing the safety of this wavelength when used on implants have been performed. Use of this wavelength, therefore, is considered inherently unsafe for implant-related procedures or periimplant surgery. Nd:YAG lasers will continue to be used successfully in periodontal therapy.16
Carbon Dioxide Lasers
The conventional CO2 laser has a wavelength of 10,600 nm. Its energy can be delivered in continuous-wave mode or gated-pulse mode and more recently, in extremely short pulses of high peak power, labeled “superpulsed” and “ultrapulsed” (i.e., ultraspeed) modes. This wavelength is highly absorbed in water, collagen, and hydroxyapatite19 and is therefore extremely efficient for soft tissue vaporization. The delivery system usually is a mirrored handpiece (making this a noncontact application) at the end of an articulated arm or a waveguide. The following discussion focuses on this wavelength of CO2 lasers and its various pulse parameters.
CO2 lasers have been used for decades in surgical procedures because of their speed and efficiency in cutting soft tissue.25 They also offer strong hemostatic and bactericidal effects and create minimal wound contraction, thereby minimizing scarring. CO2 lasers also have minimal depth of penetration, reducing lateral thermal damage.12,16 The early devices produced significant carbonization because of the high energy densities created. With the newer pulsed models, however, the energy density is reduced to between 180 and 300 mJ/cm2, delivered at an average speed of 400 to 800 μsec. These settings create less carbonization and charring of tissue and improve the working speed and efficiency of the CO2 laser. This technology has been further refined with the advent of even shorter pulsing with higher peak powers. By increasing the speed of transmission and decreasing the pulse width, the laser can cut deeper and carbonize less tissue. Thus energy density is now reduced to between 50 and 300 mJ/cm2, delivered in speeds of 30 to 80 μsec. These improvements have created an extremely versatile CO2 laser that can safely treat tissue in periodontal pockets and also make surgical incisions up to 4 to 5 mm deep rapidly and efficiently.
The CO2 laser is safe to use around implants because the energy is absorbed into water and not pigments.26,27 With its effect restricted to the intracellular water of bacteria, the CO2 wavelength can safely and effectively treat periimplantitis and mucositis,28 because the energy is not absorbed into the implant’s surface. At the same time, this laser’s hemostatic properties are excellent, allowing better visualization of the surgical field, often decreasing procedure time and limiting or preventing postoperative complications (pain, swelling).29
With the newer devices, the energy is safe when it comes into contact with bone. When exposed to CO2 laser energy, the water molecules on the surface of the bone are dehydrated, forming a thin carbon layer of approximately 0.1 mm. The resulting surface will no longer absorb energy, and the damage to bone is clinically insignificant.30 However, if the CO2 energy causes hemostasis of bony structures during surgery, curetting the bone to reestablish bleeding for healing is indicated. In my experience, the CO2 laser is the most versatile of all of the soft tissue lasers available for implant therapy.
A new wavelength of CO2 laser was recently introduced to the dental market. This wavelength of 9300 nm is delivered by means of an articulating arm. To date, no studies describing the use of this laser in periimplantitis treatment have been published; therefore great caution is advised regarding this clinical application. It is dangerous to extrapolate results with the 10.6-μm CO2 laser to justify the use of the 9.3-μm CO2 laser. For the purposes of this chapter, the only CO2 laser considered appropriate for treatment of implant/periimplant problems is the conventional 10.6-μm CO2 laser.
Erbium Lasers
The erbium family of lasers includes two similar wavelengths: the Er:YAG laser emitting at 2940 nm and the Er,Cr:YSGG laser at 2780 nm. Both lasers are operated in a free-running pulsed mode. The method of delivery is by mirrored handpiece and articulated arm, waveguide, or trunk fiber and handpiece with a quartz or sapphire fiber tip. The delivery systems include a water spray to prevent heat buildup and to rehydrate the target tissues so that the energy will be absorbed more efficiently.
The erbium wavelengths are highly absorbed in water and hydroxyapatite. They are good for ablating hard tissues such as tooth structure and bone. When first introduced to the market, the U.S. Food and Drug Administration (FDA) cleared the erbium lasers only for hard tissue procedures. By vaporizing water molecules within the hard tissues, erbium lasers create microexplosions in the hydroxyapatite that break down the hard tissue during the ablation process. This effect is achieved without charring or carbonization, and the heat generated is minimal (see Chapter 10). The erbium lasers also will ablate soft tissue, but with limitations. It is most effective in lightly vascularized tissue where bleeding will not be an issue. The erbium wavelength is the least effective of all of the dental laser wavelengths in achieving hemostasis.
Because its energy is absorbed into water, the erbium laser is safe to use around implants and can treat periimplantitis and mucositis safely.31,32 This laser will leave the bony surface bleeding (for healing), so curettage is not necessary, but it will not harm the implant’s surface.33 Erbium lasers have excellent bactericidal properties because the energy ruptures the cell membranes of bacteria when absorbed into intracellular water.
Laser Applications in Clinical Practice Preoperative Frenectomy and Tissue Ablation
In certain instances, the clinician may need to alter the soft tissue architecture adjacent to the surgical site before the implant is placed. For example, a patient with a high muscle attachment too close to the surgical site would benefit from a frenectomy, to alleviate any tension on the tissue around the implant site. The more complex the surgery, such as bone grafting with creation of a flap, the more important it is to release the muscle tension. The release of muscle tension provides a greater opportunity for success, without sutures pulling, with less postoperative pain and swelling. A frenectomy can be accomplished using any of the soft tissue lasers discussed earlier (Figure 7-2).
Before tooth extraction, the clinician also may need to alter the soft tissue if it is too thick or uneven in thickness. In Figure 7-3, by ablating 2 to 3 mm of tissue in a broad area distal and palatal to the upper right second molar, the resulting tissue thickness can then conveniently accommodate the abutment and crown with hygienically manageable architecture. The ability to remove tissue easily without bleeding, swelling, or postoperative pain is a tremendous advantage to both the clinician and the patient.
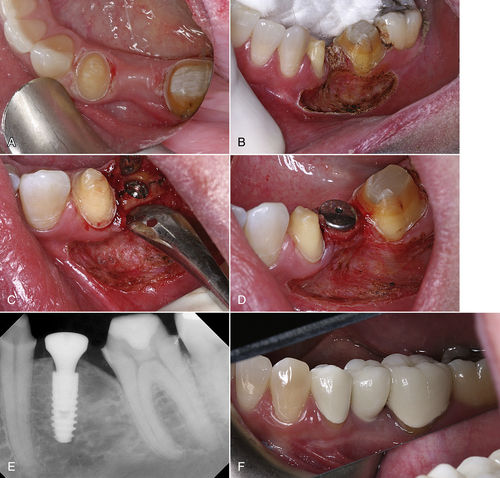
• Figure 7-2 A, Pretreatment occlusal view of planned implant surgery site. B, Immediately after muscle release and frenectomy. Note that periosteum is intact. C, Midcrestal incision performed with an ultraspeed CO2 laser and placement of the implant. D, Tissue former is placed and tissue sutured into place with two single sutures. No dressing was placed on the tissue. E, Radiograph of the implant with tissue former in place. F, Final crown seated on implant 4 months after placement.
Preparation of Surgical Site
Site preparation is the first step of implant surgery. To prevent contamination of the surgical site, clinicians have used a variety of antimicrobial rinses, including chlorhexidine, before the procedure.35,36 Such decontamination efforts have been only partially effective, however, because of the immense bacterial load in the oral cavity. Furthermore, if the site were to become contaminated with saliva during surgery, it would not be practical or effective to stop and rinse again.
Lasers present an excellent solution to the problem of surgical site contamination. All lasers are bactericidal. The clinician simply needs to expose the surgical site to the laser energy for a few seconds. The bactericidal effects are profound and almost instantaneous, and an implant site can be sterilized.37 Before osteotomy development, the soft tissue can be sterilized much more effectively with a laser than with rinsing or swabbing. Furthermore, with accidental contamination of the surgical site by saliva during the procedure, simply reapplying the laser in the area will reestablish sterility, so that the procedure can continue with the greatest chance for success. The erbium and diode wavelengths can accomplish decontamination if the contact laser physically “touches” every square millimeter of the surface to be sterilized.7,38 For this purpose, a slow, deliberate application technique is required; the larger the osteotomy site, the longer the sterilization procedure.
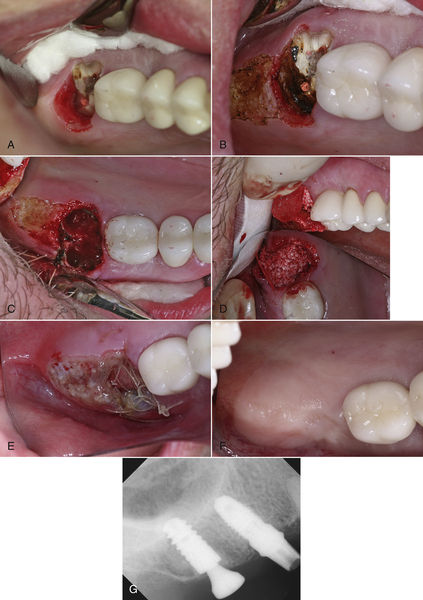
• Figure 7-3 A, Excessive tissue thickness distal and palatal to upper right second molar, which is to be extracted and replaced with an implant. B, Ultraspeed CO2 laser was used to reduce the tissue contours where indicated. C, Extraction socket immediately after extraction of root. D, Socket with osseous grafting material in place. E, Postoperative view of extraction/graft site and tissue reduction at 24 h. Note good pink color of tissue and lack of tension on sutures. F, Surgical site at 5 months postoperatively. G, Radiograph of implant with tissue former.
Decontamination and Implant Placement
A CO2 laser has a distinct advantage over contact lasers: Because CO2 lasers are noncontact, it is a simple procedure to place a wide-aperture handpiece on the CO2 laser to apply the laser beam out of focus, which would increase the spot size on the tissue even more. Sterilization of a large osteotomy site with a CO2 laser takes mere seconds. As the surgery progresses, the clinician and the assistant should be diligent in keeping the surgical site free of saliva. In most single-implant scenarios, this aim can easily be accomplished. In large-scale surgical procedures with multiple implants or large incisions, however, it may be difficult to maintain a sterile environment. When necessary, laser energy can be redirected to the tissues to decontaminate the surgical site as often as the surgeon deems necessary, using an appropriate power setting for sterilization (Figure 7-4).
Another clinical situation requiring decontamination involves extraction of teeth followed by immediate placement of implants into the extraction site. In some cases, the presence of infected tissues is obvious, and the clinician notices soft tissue around the apices of roots or in the furcation area of molars. Even if infection is not readily apparent, however, a prudent approach is to assume that it might compromise the results of the procedure. The surgical goal is to eliminate all diseased soft tissues in the extraction site and to decontaminate all bony surfaces within the site. A spoon curette can be used to remove gross amounts of soft tissue easily and quickly, with subsequent laser excision to remove any tissue tags. The entire inner surface of the extraction socket can then be decontaminated with a laser.
As with decontaminating the soft tissue of the surgical site immediately before raising the flap, it is difficult to “touch” all of the surfaces of the socket with a diode or Nd:YAG fiber. Of course, neither diode nor Nd:YAG lasers are indicated for use on bone. The erbium wavelengths are effective in removing the remaining soft tissue and decontaminating the bony surfaces at lower power settings with a water-coolant spray.13 Because they are not as effective as other wavelengths in creating hemostasis, erbium wavelengths would leave the bone surfaces bleeding, which enhances healing of the socket, whether an implant or a graft is placed, or the socket may simply be left to fill in. The CO2 lasers also are a good choice because they also will remove soft tissue tags and decontaminate bony surfaces, again at low power densities.39 However, CO2 laser energy is an excellent hemostatic wavelength, so the effect of hemostasis39 on the tissues must be overcome for healing.35
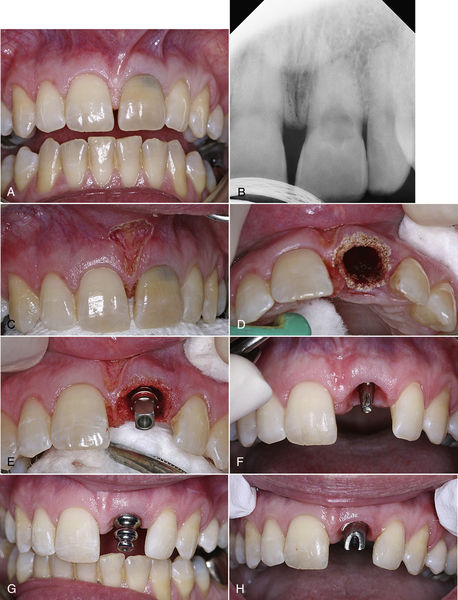
The clinician should gently curette the bone to reestablish bleeding and maximize the healing potential of the implant or graft site. The laser energy must be delivered to all of the bony surfaces within the extraction site. If a severely dilacerated root poses a barrier to the operative line of sight for access of the laser beam, the clinician should make the decision to allow the body’s natural defense mechanisms to heal the site over weeks and make it safe for reentry to perform the implant or grafting procedures. Figure 7-5 illustrates the procedure for decontaminating a surgical site and socket, involving an upper left central incisor planned for extraction. An important consideration in this scenario is the proximity of the frenum to the surgical site. A laser frenectomy is performed to ensure no tension on the tissues immediately surrounding the site. After sterilization of the bony crypt and surrounding marginal tissue, the implant is placed with confidence that the soft and hard tissues of the surgical area are free of disease and bacteria.
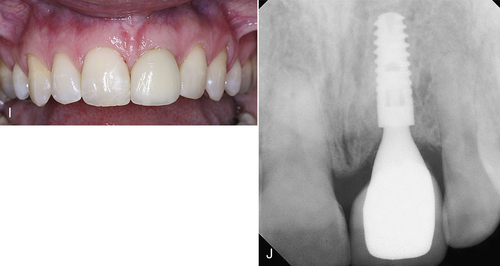
Figure 7-6 illustrates a similar situation with an upper left first premolar planned for extraction and implant placement. Both the internal and the external aspects of the surgical site are decontaminated with the laser. An abutment is placed so that the patient can wear a temporary fixed prosthesis in that quadrant. A soft tissue troughing procedure is performed on the molar. At 4 months, the soft tissue surrounding the implant is recontoured for a better esthetic result.
Osteotomy
Soft Tissue
The next objective in laser implant surgery is the preparation of the osteotomy, with different considerations for cutting through soft tissues versus hard tissues. The clinician must first decide on the desired pattern of entry through the soft tissue. In some cases a minimal entry, often referred to as a “punch procedure,” is the goal. The soft tissue is removed as a 3- to 4-mm-diameter “plug” down to the crest of bone. This soft tissue may be 1 to 2 mm or 3 to 4 mm thick, depending on location and biotype. If the tissue is relatively thin (1 to 2 mm), any wavelength is acceptable. If the tissue is thicker, using a diode or Nd:YAG laser may take minutes versus seconds for erbium and CO2 lasers. Depending on the tissue quality, bleeding may be an issue with erbium lasers. By quickly and efficiently cutting through the tissue and creating optimal visibility for the surgeon, the duration of the procedure often may be reduced compared with that for conventional techniques.
Other designs for tissue entry include small envelope flaps, often used to gain tissue height (as in anterior implants and other areas) and to provide better-quality tissue around the abutment-crown complex (Figure 7-7).
As the entry site increases in size and the flap design becomes more complex, going through multiple layers of both attached (keratinized) and nonattached (mucosal) tissues becomes a significant consideration in choosing the proper wavelength. Speed of cutting reduces procedure time, as does hemostasis, which also enhances vision. Thus, as more soft tissue is involved, diode and Nd:YAG lasers become less effective—these are contact lasers, so to cut through larger amounts and more layers of soft tissue, more time is required to make the incision. Erbium lasers do not provide hemostasis as well as other wavelengths for larger incisions. The unobstructed vision, excellent hemostasis, and efficiency of cutting through all tissue biotypes and thicknesses make the CO2 laser most suitable for these procedures.39
Laser Advantages
Using laser energy to make any incision has several benefits. First, a sterile cut is less likely to become infected. Lasers incise tissue without creating the cascade of events that leads to swelling and inflammation. Because lasers seal off lymphatics and blood vessels, a clinically measurable reduction in pain, swelling, and other postoperative complications has been documented for these incisions.40,41 With reduced swelling, sutures will not pull through the tissue or are less likely to come undone. Analgesics and antibiotics are needed less frequently, and often in less potent formulations (with fewer drug interactions), because patients experience a significantly less traumatic postoperative course. These benefits apply for both minor and major surgical procedures.
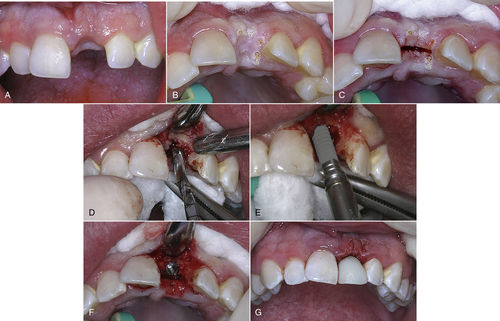
• Figure 7-4 A, Preoperative photograph of implant site for replacing upper left central incisor. B, Surgical site decontaminated with an ultraspeed CO2 laser. C, Midcrestal incision made with the laser. D, The flap is elevated and the osteotomy site is being prepared. Note excellent visualization of the surgical site with no bleeding to obscure the surgeon’s vision. E, Implant being placed into osteotomy site. F, Completed implant placement. G, Immediate temporization of implant with abutment and temporary crown, and tissue reapproximated with two single sutures.
Hemostasis
Another advantage of laser use is the relative safety of such approaches in patients who are anticoagulated with common medications such as aspirin, clopidogrel (Plavix), and warfarin (Coumadin). Some patients also take herbal remedies that can significantly alter their clotting time. The main question with anticoagulated patients is whether their medication should be stopped before surgery. The clinician needs to be aware of the individual patient’s circumstances and consult with the primary care physician. Before any dental surgery, the patient’s health history must be reviewed and updated. With any concern regarding the patient’s medications, the appropriate laboratory work, including an international normalized ratio (INR), must be ordered. Recent studies on altering a patient’s medications before dental surgery reveal few if any reasons to change the anticoagulant regimen if the INR is less than 4.0,42,43 although the final decision rests with the primary care physician. Patients receiving anticoagulant therapy will benefit more from the use of lasers in dental surgical procedures than healthier patients. Most lasers have excellent hemostatic properties that lead to decreased bleeding, so intraoperative hemorrhage control is less of an issue.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses