For many intraoral soft-tissue surgical procedures the laser has become a desirable and dependable alternative to traditional scalpel surgery. However, the use of dental lasers in periodontal therapy is controversial. This article presents the current peer-reviewed evidence on the use of dental lasers for the treatment of chronic periodontitis.
For many intraoral soft-tissue surgical procedures the laser has become a desirable and dependable alternative to traditional scalpel surgery. The dental literature contains many case reports and uncontrolled case studies that report on the use of various laser wavelengths, predominantly diode, CO 2 , Nd:YAG, Er:YAG, and Er, Cr:YSGG, for various intraoral soft-tissue procedures, such as frenectomy, gingivectomy and gingivoplasty, de-epithelization of reflected periodontal flaps, second stage exposure of dental implants, lesion ablation, incisional and excisional biopsies, irradiation of aphthous ulcers, removal of gingival pigmentation, and soft-tissue crown lengthening. Lasers easily ablate and reshape oral soft tissues. In addition, lasers increase hemostasis through heat-induced coagulation and occlusion of arterioles, venules, and capillaries. The resulting hemostasis allows for a clear and fully visible surgical field. Because of the intense heat, lasers also have the advantage of a bactericidal effect at the target site. A few studies have reported that laser surgery, compared with traditional scalpel surgery, is less painful, features less swelling, and heals faster with less wound contraction. However, there are conflicting opinions on pain and speed of wound healing. Several papers comparing lasers with traditional scalpel wounding have reported either an equivalent effect or that laser surgery is accompanied by more pain and slower healing. The issues of pain and wound healing, and wound contraction seem dependent on the judicious choice of parameters such as power, hertz, pulse duration, and time of exposure.
Given the apparent usefulness of the laser, why, after almost 2 decades, does the use of dental lasers in periodontal therapy remain controversial? Is it because lasers challenge the traditional modalities of treating periodontitis or because of a lack of hard evidence on which to make an informed decision? One may argue in favor of one or both of these reasons. It is well known that many in private practice are using various types of lasers for the treatment of periodontal disease and most have expressed satisfaction with the results of therapy. However, several recent systematic reviews of the literature have suggested there is little evidence in support of the purported benefits of lasers in the treatment of periodontal disease compared with traditional periodontal therapy. The obvious question then becomes, is the current use of dental laser for the treatment of periodontitis based on peer-reviewed published evidence obtained under controlled conditions or word-of-mouth, unconfirmed evidence?
A letter to the editor in a recent issue of the Journal of Dental Education asked the following question: “Why is it that dentists are among the very few health professionals who can ignore critical evaluation of the scientific literature and treat patients with personal experience as its equal?” The authors suggest that many dentists may be providing treatment without critically evaluating whether such treatment is consistent with the best evidence.
The authors also present several possible reasons for ignoring the best available evidence, such as expediency, difficulty finding reliable evidence-based references, easy access to questionable information, and a desire for quick profits. Other reasons may include the introduction of new products without rigorous clinical trials. Regulatory agencies such as the US Food and Drug Administration (FDA) do not necessarily require clinical research before product marketing. As an example, in the case of dental lasers, the 510K FDA premarket notification process requires only that the applicant provide evidence that its device is substantially equivalent to 1 or more similar devices currently marketed in the US marketplace. A 510K premarket notification does not imply therapeutic equivalency or superiority. Indeed, the 510K process does not even require a clinical trial.
Given the current conflicting opinions, this article presents the current peer-reviewed evidence on the use of dental lasers for the treatment of chronic periodontitis.
Wavelength
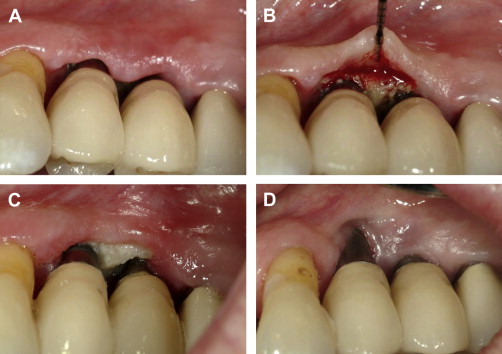
Wavelength can be related to collateral tissue damage. In general, the shorter wavelength lasers (eg, 809 nm to 980 nm diodes and 1064 nm Nd:YAG) are more likely to penetrate deeper into soft tissues. The extent of tissue penetration by shorter wavelengths is related to their affinity for pigmented tissues and a low absorption coefficient in water. The potential for undesired tissue penetration can be controlled with proper selection of parameters, such as power level, pulse repetition rate, pulse width, and energy density. In contrast, the longer wavelengths (2940 nm Er:YAG, 2780 nm Er, Cr:YSGG, and 10,600 nm CO 2 ) show comparatively more shallow tissue penetration because of their high absorption coefficients in water. To avoid unintended consequences, the least amount of power required to achieve the desired clinical result should be chosen. Hard tissues and periosteum subjacent to thin oral mucosa (thin biotype), the gingival margin, or gingiva overlying prominent roots, are particularly vulnerable to thermal insult ( Fig. 1 A–D). The potential for damage to dental hard tissues results from uncontrolled penetration during soft-tissue procedures that, in turn, results from improper choice of parameters while using a short wavelength laser or the conductive heat effects arising from superheating of char produced by longer wavelengths. Moreover, only the erbium family of lasers have a specific indication for use on dental hard tissue, and other wavelengths should be avoided. The clinician is well advised to assess tissue thickness before initiating any laser procedure involving the gingival sulcus or the periodontal pocket area.