Periodontal disease is treated by various approaches, including simple oral hygiene practices, professional mechanical debridement, antimicrobial therapy and periodontal surgery. There is evidence to associate periodontal disease with several systemic diseases and conditions, including myocardial infarction, adverse pregnancy outcomes, diabetes mellitus, and respiratory disease. This article reviews the published literature that describes the effects of periodontal treatment on cardiovascular diseases, adverse pregnancy outcomes, diabetes mellitus, and respiratory disease. While some progress has been made, further research is required to understand the value of periodontal interventions in the prevention of systemic diseases.
As detailed in the previous articles in this issue, periodontal disease is treated by various approaches, from simple oral hygiene practices and professional mechanical debridment, and antimicrobial therapy, to periodontal surgery that may involve the use of bone grafts, guided tissue regeneration, growth factors and beyond. Treatment is almost always rendered to manage the local effects of periodontitis, that is the localized destruction of the tooth attachment apparatus by inflammation induced by the bacteria of dental plaque.
In the past two decades evidence has emerged associating periodontal disease with several important systemic diseases and conditions. Results from numerous case-control and epidemiologic studies suggest that people with periodontal disease have a modestly higher risk for myocardial infarction (MI) when compared with people without periodontal disease. Other studies have also connected periodontal disease with adverse pregnancy outcomes, diabetes mellitus, various lung diseases such as pneumonia and chronic obstructive lung disease, and Alzheimer’s disease. In the future, there may be additional justification for such therapy should periodontal disease be proven to influence the initiation or progression of systemic diseases such as atherosclerosis, adverse pregnancy outcomes, diabetes mellitus, or chronic lung disease.
What is the evidence for the efficacy of periodontal therapy in the treatment or prevention of systemic disease? A complete review of the literature that addresses all evidence for or against a role for periodontal disease in the pathogenesis of systemic diseases is beyond the scope of the present article. Instead, the published literature that describes the effects of periodontal treatment on cardiovascular diseases (CVDs), adverse pregnancy outcomes, diabetes mellitus, and respiratory disease is reviewed.
CVD
Although it is unknown how periodontal disease influences the course of atherosclerosis, several mechanisms have been proposed involving direct effects of periodontal bacteria that injure vascular tissue, and indirect effects mediated through innate and specific immune responses against periodontal bacteria causing vasculature damage. Regardless of mechanism, it has been assumed that periodontal treatment would reduce the insult imposed by the microorganisms of dental plaque to reduce the inflammatory response and thus atherosclerosis.
In July 2009 a computer search using the following search terms was conducted using PubMed: periodontal; treatment; cardiovascular; randomized; and trial. A total of twenty one articles were retrieved. Although no studies have been published that measure the effect of periodontal treatment on a clinical outcome such as MI, seven papers were chosen for review that describe a well-designed randomized trial that measured surrogate markers believed to serve as risk indicators for MI. Risk indicators measured included acute-phase response markers such as C-reactive protein (CRP), fibrinogen, serum α-amyloid A, and proinflammatory cytokines such as interleukin 1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) ( Table 1 ). In some cases, flow-mediated vascular dilatation was measured.
| Citations | Number of Subjects (Placebo/Control) | Treatment | Outcomes Measured | Results | Comments |
|---|---|---|---|---|---|
| Ide et al | 39 nonsmoking subjects with moderate to advanced chronic periodontitis. Random assignment to immediate treatment or treatment following a 3-month non-treatment phase | Full-mouth scaling and root planing | Dental outcomes: plaque index, bleeding on probing, pocket depths. CVD outcomes: serum levels of CRP, fibrinogen, serum α-amyloid A, IL-1β, IL-6, and TNF-α | Treatment significantly improved periodontal status. No significant changes in levels of any of the systemic markers were noted | Small number of patients examined |
| D’Aiuto et al | 40 systemically healthy subjects with severe chronic generalized periodontitis. Random assignment to either standard treatment (20 subjects) or intensive treatment (20 subjects) and followed for 6-month trial | SPT: only full-mouth scaling and root planing of all teeth. IPT: full-mouth scaling and root planing of all teeth plus adjunctive local delivery of minocycline microspheres (Arestin, OraPharma, Warminster, PA) | Dental outcomes: average full-mouth supragingival plaque scores, average full-mouth gingival bleeding scores, and average full-mouth number of periodontal lesions. CVD outcomes: serum CRP, IL-6, leukocyte counts, HDL-C, BP, body mass index, Framingham risk scores | Both treatments improved periodontal status compared with baseline. IPT showed significant reductions in inflammatory markers (IL-6 and CRP) and lipid markers (TC and LDL-C) at 2 and 6 months, no changes were observed for systemic markers in the SPT group. Both treatments reduced leukocyte counts at 6 months. BP reduced after 2 months by IPT. | Small number of patients examined; all subjects had severe, generalized periodontitis |
| Mercanoglu et al | 54 subjects were enrolled – 28 with chronic periodontitis and without atherosclerotic disease, and 26 healthy controls. Subjects with periodontitis were compared with subjects with healthy periodontium; all subjects received the same treatment | Scaling and root planning of all teeth | Dental outcomes: PI, GI, PD, and CAL, radiographic bone levels. CVD outcomes: flow-mediated dilatation of the brachial artery following transient ischemia | Periodontal therapy significantly reduced mean PI, GI, PD, and CAL of the periodontitis group. Increases in brachial artery diameter induced by reactive hyperemia and sublingual nitroglycerine in the periodontitis group were significantly less than the control group at baseline. There was no significant difference between the initial and final measurements of flow-mediated dilatation in the control subjects. After periodontal treatment, the changes in brachial artery diameter induced by reactive hyperemia and sublingual nitroglycerine in the posttreatment measurements were significantly higher when compared with initial measurements | Endothelial functions in patients with chronic periodontitis were impaired compared with subjects without periodontitis. Recovery of endothelial dysfunction after periodontal treatment was demonstrated. Small number of patients examined. |
| D’Aiuto et al | 65 subjects with severe periodontitis. Random assignment to untreated control group (24 subjects); standard periodontal treatment group (SPT: 21 subjects), or an intensive periodontal treatment (IPT: 20 subjects), consisting of SPT plus local minocycline hydrochloride for 2 months | SPT: only full-mouth scaling and root planing of all teeth. IPT: full-mouth scaling and root planing of all teeth plus adjunctive local delivery of minocycline microspheres (Arestin, OraPharma, Warminster, PA) | Dental outcomes: percentage of tooth sites with dental plaque, percentage of tooth sites with bleeding on probing, full-mouth average periodontal pocket depth, full-mouth average clinical attachment loss. CVD outcomes: CRP, IL-6, total cholesterol, and LDL-C | Periodontal therapy (either standard or intensive) resulted in an additional reduction in serum CRP of at least 0.5 mg/L compared with the untreated controls | SPT and IPT resulted in statistically significant reductions of periodontal lesions after therapy. No changes were observed in the untreated controls |
| Seinost et al | 30 patients with severe periodontitis and 31 control subjects were assessed FMD of the brachial artery | All patients received oral hygiene instructions, scaling and root planing of all teeth. CHX (0.1%) mouth rinses for 14 days, and systemic amoxicillin plus clavulanic acid and metronidazole therapy was administered for 7 days. Residual pockets of more than 5 mm that bled after probing were rescaled 12 weeks after periodontal treatment | Dental outcomes: bleeding on probing, probing depth, gingival recession. CVD outcomes: measurement of serum total cholesterol, triglycerides, HDL, Hb A 1c , LDL-C, CRP. Brachial artery reactivity was assessed within 1 week of the initial treatment and 3 months after end of treatment | Subjects with periodontitis had significantly higher baseline levels CRP than controls. After periodontal treatment, CRP significantly decreased. FMD expressed as change in diameter and as percent change was significantly lower in patients with periodontitis before treatment than in healthy controls after 3 months. Nitroglycerin-associated dilation did not differ significantly between controls and patients with periodontitis before and after treatment | |
| Tonetti et al | 120 patients with severe periodontitis. Random assignment of 59 subjects to community-based periodontal care/61 to intensive periodontal treatment followed for 6 months | Full-mouth scaling and root planing; extraction of hopeless teeth; local placement of microspheres of minocycline into periodontal pockets | Dental outcomes: dental plaque score, gingival bleeding on probing score, periodontal pocket depth, gingival recession, CVD outcomes: endothelial function by FMD of the brachial artery, serum CRP, IL-6, soluble E-selectin, t-PA, PAI-1, and von Willebrand factor | Significantly greater FMD in the treatment group compared with control-treatment after 60 days. Plasma levels of soluble E-selectin were lower in the intensive-treatment group than in the control group | |
| Offenbacher et al | 303 subjects with periodontal disease and a history of blockage of 1 coronary artery or, a coronary event, including MI, coronary artery bypass graft surgery, or coronary transluminal angioplasty with or without a stent. Random assignment of 152 subjects to community-based periodontal care/151 to periodontal treatment | Full-mouth scaling and root planing | Dental outcomes: periodontal probing depth, bleeding on probing, and periodontal attachment level, GCF IL-1β. CVD outcomes: serum hs-CRP | Improved periodontal status in treatment group (pocket reduction). No reduction in GCF IL-1β or serum hs-CRP concentrations in treatment group compared with community controls | Obesity and periodontal treatment in some community controls may have confounded data interpretation |
Although most of these trials showed reduction in one or more surrogate markers for atherosclerosis activity following periodontal therapy, several trials showed no effect.
Most of these trials are small, enrolling between 30 and 65 patients, with the exception of the study of Tonetti and colleagues, which enrolled 120 patients, and the Periodontal and Vascular Events (PAVE) study, which enrolled 303 patients. Typically, the active treatment involved professional mechanical debridment compared with a control group that received the treatment at the end of the trial after measurement of outcomes. Treatment sometimes included periodontal surgery or systemic or local administration of antibiotics. In some cases the control group were untreated periodontal patients, or patients who received community care versus purposeful intervention by a study investigator.
The available results must be interpreted with caution. The lack of an accepted standard for treatment of periodontitis presents considerable heterogeneity in results. Variations in treatment (eg, scaling and root planing (SRP) alone, with or without surgery, with or without antibiotics) yield results that may be difficult to interpret. Should patients receive only one course of local debridment, or should they be treated until they demonstrate an absence of periodontal inflammation? These different periodontal end points would greatly modify study design and the cost and duration of a clinical trial. The sample size of most of the studies may be underpowered to detect all but robust treatment effects. Because the primary outcome of interest (MI) is of low prevalence in most populations, large sample sizes are required to detect differences in treatment arms should such differences exist. Such studies are expensive to run. The length of trials may be too brief in duration to observe meaningful outcomes. Indeed, clinical outcomes of processes such as atherosclerosis (eg, MI) are often years in the making. Perhaps a short-term periodontal treatment might not have a discernable effect on such long-term clinical outcomes.
Adverse pregnancy outcomes
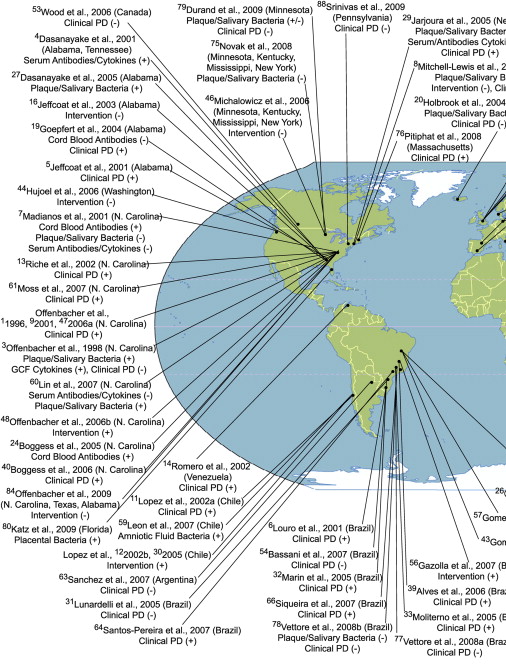
Periodontal disease is related to increased inflammatory mediators and prostaglandins and some of these molecules such as prostaglandin E 2 (PGE 2 ) are known to influence preterm delivery. In this regard, it is possible that pregnant women with periodontal disease experience more preterm deliveries. Before evaluating the results of periodontal disease treatment trials in relation to poor pregnancy outcomes, it is useful to understand the existing body of literature that links periodontal disease with poor pregnancy outcomes. To establish such an association using observational studies, clinical, bacteriologic, immunologic, and inflammatory mediator outcomes related to periodontal disease must be measured without any errors and their independent effect on poor pregnancy outcomes should be measured, using either a prospective, case-control, or cross-sectional studies with adequate numbers of subjects, while taking into account potential confounding factors. Measurement of elements related to periodontal disease locally and systemically, and specifically within the fetoplacental unit, and within a time period that makes biologic sense in relation to pregnancy outcomes, would validate observational studies. As of October, 2009, 88 studies from populations around the world have been published that address the potential link between parameters related to periodontal disease and poor pregnancy outcomes such as low birth weight, preterm birth, and preterm low birth weight. These studies, their location, the periodontal parameters that were evaluated, and the nature of the association of these parameters with poor pregnancy outcomes (positive association [+] or no association [−]) are depicted in Fig. 1 . Fifty of these studies reported a significant association, although 24 studies failed to find an association.
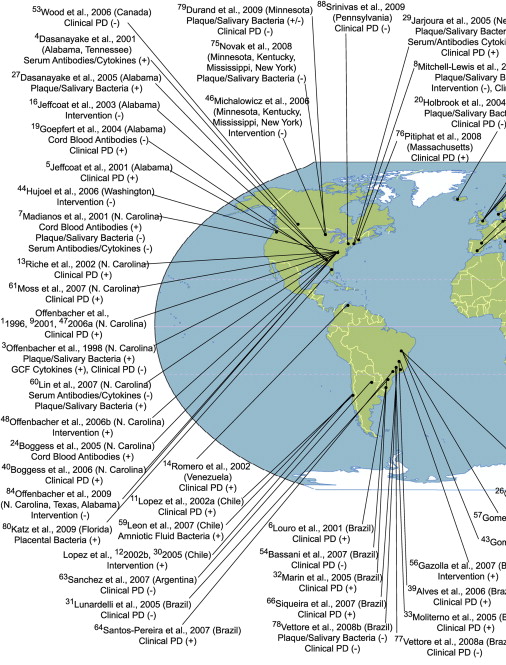
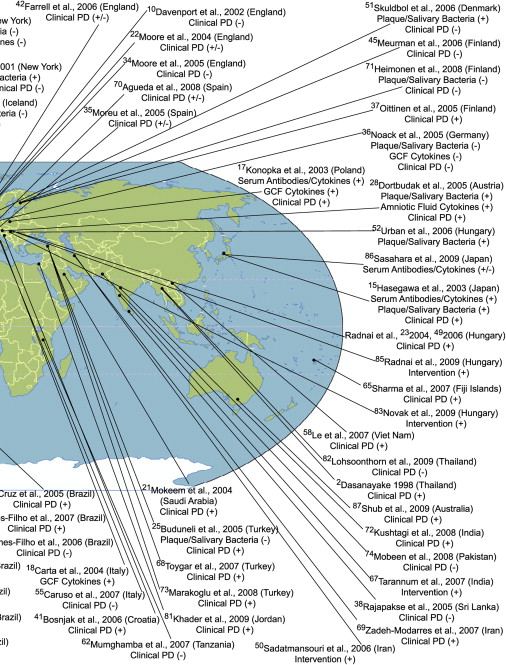
Such a depiction, however, does not take into account the inherent strengths or the limitations of these studies. One way to evaluate the association between periodontal disease and pregnancy outcomes is to pool the results from these studies, while taking into account their strengths and weaknesses. Meta-analysis of existing studies allows this to some extent. Based on 17 observational studies, Vergnes and Sixou concluded that the overall association between periodontal disease and preterm low birth weight was statistically significant (overall odds ratio [OR] = 2.83; 95% confidence interval [CI] = 1.95–4.10). However, this may not be accurate either as some studies suffer from residual confounding caused by inadequately controlled factors such as smoking. One way to remove this “threat” is to conduct a study within a population in which pregnant women do not engage in risk behaviors such as smoking, alcohol consumption, and drug use. As these behaviors are causally related to poor pregnancy outcomes and are also related to periodontal disease, by removing the effect of such factors from the association between periodontal disease and poor pregnancy outcomes, the credibility of this association can be enhanced. By studying Sri Lankan women who do not engage in these behaviors because of economic and cultural barriers, in a prospective study it has been reported that the unconfounded association between clinical periodontal disease and preterm delivery is not statistically significant.
Definitive evidence to test this association, however, could come from large randomized clinical trials, as they are generally not subjected to the limitations of observational studies discussed earlier. By randomly allocating sufficient numbers of patients to treatment and control groups, a better control of potential confounding factors and biases can be achieved. However, the results from randomized trials are equivocal. Whereas studies conducted in Chile suggest that periodontal treatment given to pregnant women with gingivitis or periodontitis results in a reduction of preterm low birth weight, studies conducted in the United States failed to observe similar results. It is unlikely that this discrepancy is caused by population differences. Whereas the Chilean studies provided mechanical periodontal treatment as needed during pregnancy, the US studies were restricted to a limited number of scaling and root planning sessions. However, careful review of one of the US studies, suggests that other pregnancy outcomes such as spontaneous abortion and stillbirth were significantly reduced in the treatment group. In a treatment study in which metronidazole was used, Jeffcoat and colleagues observed an increased risk of preterm birth in the treatment group. All studies have shown that periodontal treatment rendered during pregnancy was safe.
Among the potential explanations for these discrepancies are inappropriate timing of treatment, inadequacy of treatment, and limitations in the selected outcomes. Despite these concerns, when data from seven of these randomized trials were pooled using meta-analysis, Polyzos and colleagues showed that periodontal treatment during pregnancy significantly reduced preterm birth (OR = 0.55; 95% CI = 0.35–0.86) but not low birth weight (OR = 0.48, 95% CI = 0.23–1.0).
In conclusion, the “jury is still out” on the role of periodontal disease in poor pregnancy outcomes. A definitive, adequately powered human experimental study is required that demonstrates a correlation between conversion of poor periodontal status to health in the treatment group for the duration of pregnancy, with a parallel reduction in adverse pregnancy outcomes. Until then, it can be concluded that periodontal treatment is safe for pregnant women, and that women should maintain good periodontal health before, during, and after pregnancy.
Adverse pregnancy outcomes
Periodontal disease is related to increased inflammatory mediators and prostaglandins and some of these molecules such as prostaglandin E 2 (PGE 2 ) are known to influence preterm delivery. In this regard, it is possible that pregnant women with periodontal disease experience more preterm deliveries. Before evaluating the results of periodontal disease treatment trials in relation to poor pregnancy outcomes, it is useful to understand the existing body of literature that links periodontal disease with poor pregnancy outcomes. To establish such an association using observational studies, clinical, bacteriologic, immunologic, and inflammatory mediator outcomes related to periodontal disease must be measured without any errors and their independent effect on poor pregnancy outcomes should be measured, using either a prospective, case-control, or cross-sectional studies with adequate numbers of subjects, while taking into account potential confounding factors. Measurement of elements related to periodontal disease locally and systemically, and specifically within the fetoplacental unit, and within a time period that makes biologic sense in relation to pregnancy outcomes, would validate observational studies. As of October, 2009, 88 studies from populations around the world have been published that address the potential link between parameters related to periodontal disease and poor pregnancy outcomes such as low birth weight, preterm birth, and preterm low birth weight. These studies, their location, the periodontal parameters that were evaluated, and the nature of the association of these parameters with poor pregnancy outcomes (positive association [+] or no association [−]) are depicted in Fig. 1 . Fifty of these studies reported a significant association, although 24 studies failed to find an association.