The concept that only fibroblasts from the periodontal ligament or undifferentiated mesenchymal cells have the potential to re-create the original periodontal attachment has been long recognized. Based on this concept, guided tissue regeneration has been applied with variable success to regenerate periodontal defects. Quantitative analysis of clinical outcomes after guided tissue regeneration suggests that this therapy is a successful and predictable procedure to treat narrow intrabony defects and class II mandibular furcations, but offers limited benefits in the treatment of other types of periodontal defects.
The ultimate goal of periodontal therapy is to prevent further attachment loss and predictably restore the periodontal supporting structures that were lost because of disease or trauma in a way that the architecture and function of the lost structures can be reestablished. Conventional nonsurgical therapy and periodontal flap procedures successfully halt the progression of periodontal disease but result in soft tissue recession that leads to poor esthetics in the anterior dentition. Moreover, conventional periodontal therapy often results in residual pockets usually inaccessible to adequate cleaning, which negatively affect the long-term prognosis of the treated tooth. These compromised outcomes can be avoided or minimized by periodontal regenerative procedures that restore the lost periodontal structures.
Successful periodontal regeneration relies on the re-formation of an epithelial seal, deposition of new acellular extrinsic fiber cementum and insertion of functionally oriented connective tissue fibers into the root surface, and restoration of alveolar bone height. The concept that the cells that repopulate the exposed root surface after periodontal surgery define the nature of the attachment that will form was extensively investigated. Therefore, the major factor believed to prevent periodontal regeneration after conventional therapeutic approaches is the migration of epithelial cells into the defect area at a faster rate than that of mesenchymal cells, which leads to the formation of a long junctional epithelium and the prevention of the formation of a new attachment apparatus over the previously diseased root surface. Gingival connective tissue cells can also populate the space adjacent to the denuded root surface after conventional periodontal treatment. Repopulation of the exposed root surface by gingival connective tissue cells is speculated to result in the formation of a connective tissue attachment followed by root resorption. Based on this speculation, the goal of regenerative procedures is to prevent apical migration of gingival epithelial and connective tissue cells and to provide maintenance of a wound space into which a selective population of cells (hence guided tissue regeneration [GTR]) is allowed to migrate, favoring the formation of a new periodontal attachment.
Biologic basis of GTR
GTR has successfully shown to prevent the migration of epithelial and gingival connective tissue cells into previously diseased root surfaces. The biologic basis of GTR is based on the assumption that the placement of physical barriers prevents apical migration of the epithelium and gingival connective tissue cells of the flap and provides a secluded space for the inward migration of periodontal ligament cells (PDL) and mesenchymal cells on the exposed root surface, which in turn promote periodontal regeneration. Besides favoring selective repopulation of the wound area, physical barriers are also thought to provide protection of the blood clot during the early phases of healing and to ensure space maintenance for ingrowth of a new periodontal apparatus. GTR membranes, as physical barriers, however, provide no biologic effects on differentiation and proliferation of mesenchymal and PDL cells, which is likely to limit their clinical efficacy.
Types of barrier membranes
Since the discovery that only selected cells have the potential to re-create a new periodontal attachment, a wide range of materials, including methylcellulose acetate, expanded polytetrafluoroethylene (ePTFE) (GORE-TEX, Gore, Flagstaff, AZ, USA), collagen, polyglycoside synthetic polymers, and calcium sulfate were tested for effectiveness and used as a physical barrier in GTR. These membranes are derived from a variety of sources, natural and synthetic, and are either bioabsorbable or nonresorbable.
Nonresorbable Membranes
Nonresorbable membranes, made of methylcellulose acetate (Millipore, Bedford, MA, USA), were successfully used in the first GTR case. However, these membranes were quite fragile and often tended to tear, which limited their clinical use. Methylcellulose acetate barriers were later replaced by nonresorbable ePTFE membranes (GORE-TEX) specifically designed for periodontal regeneration. Most of the current understanding regarding GTR derives from early studies using ePTFE membranes.
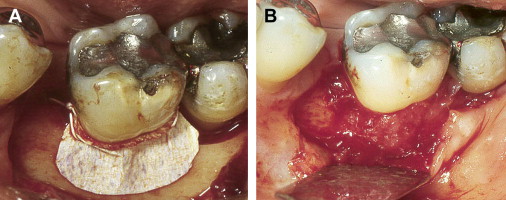
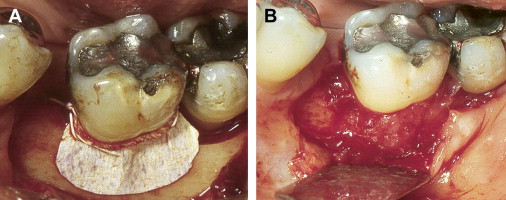
ePTFE is a synthetic biocompatible polymer consisting of a long carbon backbone to which fluorine atoms are bonded. This membrane is composed of an inner cell occlusive area and an outer cell adherent region. Because of this particular configuration, ePTFE membranes can selectively exclude migration of epithelial and gingival connective tissue cells and integrate with the bone and connective tissue margin of the periodontal defect. This material not only possesses adequate stiffness to allow creation and maintenance of a secluded space into which the new attachment will form but also is supple enough to allow adequate adaptation over the defect. ePTFE membranes are available in various configurations and sizes, in nonreinforced and titanium-reinforced configurations. Titanium-reinforced configurations are specially indicated when the defect anatomy is not supportive, such as in 1-wall defects. Although excellent clinical results have been shown with the use of ePTFE membranes, their use is associated with high frequency of early spontaneous exposure to the oral environment, which compromises their effectiveness. Moreover, these membranes need to be removed after 6 to 8 weeks in a second surgical procedure ( Fig. 1 ).
Bioabsorbable Membranes
Bioabsorbable membranes have been developed primarily to avoid a second surgery for membrane removal. Various bioabsorbable materials, including polyglycoside synthetic polymers (ie, polymers of polylactic acid, polyglycolic acid, polylactate/polygalactate), collagen, and calcium sulfate have been frequently used in membrane barriers. Similar to ePTFE membranes, resorbable membranes are biocompatible and exert their function by excluding undesirable cells from migrating into periodontal defects and providing a space for ingrowth of periodontal attachment. The clinical efficacy of bioabsorbable membranes depends on their ability to retain their structural physical integrity during the first 6 to 8 weeks of healing and to be gradually absorbed thereafter. Based on this concept, chemicals and structural modifications (ie, polymerization, cross-linking) were incorporated into bioabsorbable membranes to extend their absorption time and increase the clinical effectiveness of these materials. However, the prolonged collagen resorption rate does not always result in greater periodontal regeneration. It is possible that membranes are only required to maintain their physical integrity for 6 weeks and prolonged retention after that is detrimental to the healing process.
Collagen barrier membranes, made primarily from bovine and porcine type I collagen, were successfully used in the treatment of periodontal defects. Collagen membranes are particularly suitable for GTR applications, as collagen is chemotactic and stimulates proliferation of fibroblasts, acts as a barrier for migrating epithelial cells, provides hemostasis, serves as a fibrillar scaffold for vascular and tissue ingrowth, can be easily shaped, and is readily adaptable. These membranes are resorbed by the enzymatic activity of macrophages and neutrophils.
Degradable polymers constitute a second major group of bioabsorbable barrier membranes. These membranes are formed by copolymerization of polylactic acid, polyglycolic acid, and polylactate/polygalactate. These synthetic membranes remain intact for 20 weeks or more, depending on their polymeric composition. Afterward they are degraded by hydrolysis of ester bonds and eliminated through metabolic pathways as carbon dioxide and water. Although these membranes are considered biocompatible, their hydrolysis is accompanied by a local inflammatory response. The inflammatory response is not considered harmful, but it remains to be established as to what extent it may affect the regeneration of periodontal tissues.
Calcium sulfate can act as an exclusion barrier and has been used as a GTR membrane in the treatment of periodontal defects. This material provides good adaptation to the margins of the periodontal defect and absorbs in about 30 days without triggering an inflammatory or foreign body reaction. Unfortunately, most of the data regarding the use of calcium sulfate in GTR come from either case reports or studies with a relatively small number of cases, in which calcium sulfate was used in association with bone grafting materials, preventing an adequate assessment of its clinical efficacy.
Numerous complications, such as early membrane degradation and epithelial downgrowth, were associated with the use of bioabsorbable barrier membranes. Nevertheless, a meta-analysis of intrabony and furcation defect studies investigating open flap debridement versus GTR revealed that most of the bioabsorbable and nonresorbable GTR membranes provide superior clinical results than open flap debridement alone ( Table 1 ). But only a few studies have directly compared the effectiveness of bioabsorbable membranes with open flap debridement as a control. Newer studies comparing the use of bioabsorbable membranes with nonresorbable ePTFE membranes indicate that both membranes are equally effective in the treatment of intrabony and furcation defects. The clinical indication of nonresorbable and bioabsorbable membranes necessitates consideration of the anatomy of the periodontal defect. Therefore, nonresorbable reinforced membranes and bioabsorbable membranes supported by filler materials are used for the treatment of nonsupportive defects, such as in wide 1- or 2-walled periodontal defects. On the other hand, narrower 2-walled defects can be treated with bioabsorbable membranes.
| Barrier | Intrabony Defects (mm) (Mean ± SD) | Furcation Defects (mm) (Mean ± SD) | |||
|---|---|---|---|---|---|
| CAL | PD | VPAL | VPD | HOPA | |
| Collagen | 0.95 ± 0.47 | 1.06 ± 0.37 | 0.1 ± 0.60 | −0.04 ± 0.52 | 0.96 ± 0.91 |
| Polymers | 0.92 ± 0.18 | 0.89 ± 0.14 | 2.5 ± 0.85 | 2.30 ± 0.74 | Insufficient data |
| ePTFE | 1.62 ± 0.25 | 1.41 ± 0.2 | 1.39 ± 0.36 | 1.01 ± 0.31 | 0.99 ± 0.31 |
Types of barrier membranes
Since the discovery that only selected cells have the potential to re-create a new periodontal attachment, a wide range of materials, including methylcellulose acetate, expanded polytetrafluoroethylene (ePTFE) (GORE-TEX, Gore, Flagstaff, AZ, USA), collagen, polyglycoside synthetic polymers, and calcium sulfate were tested for effectiveness and used as a physical barrier in GTR. These membranes are derived from a variety of sources, natural and synthetic, and are either bioabsorbable or nonresorbable.
Nonresorbable Membranes
Nonresorbable membranes, made of methylcellulose acetate (Millipore, Bedford, MA, USA), were successfully used in the first GTR case. However, these membranes were quite fragile and often tended to tear, which limited their clinical use. Methylcellulose acetate barriers were later replaced by nonresorbable ePTFE membranes (GORE-TEX) specifically designed for periodontal regeneration. Most of the current understanding regarding GTR derives from early studies using ePTFE membranes.
ePTFE is a synthetic biocompatible polymer consisting of a long carbon backbone to which fluorine atoms are bonded. This membrane is composed of an inner cell occlusive area and an outer cell adherent region. Because of this particular configuration, ePTFE membranes can selectively exclude migration of epithelial and gingival connective tissue cells and integrate with the bone and connective tissue margin of the periodontal defect. This material not only possesses adequate stiffness to allow creation and maintenance of a secluded space into which the new attachment will form but also is supple enough to allow adequate adaptation over the defect. ePTFE membranes are available in various configurations and sizes, in nonreinforced and titanium-reinforced configurations. Titanium-reinforced configurations are specially indicated when the defect anatomy is not supportive, such as in 1-wall defects. Although excellent clinical results have been shown with the use of ePTFE membranes, their use is associated with high frequency of early spontaneous exposure to the oral environment, which compromises their effectiveness. Moreover, these membranes need to be removed after 6 to 8 weeks in a second surgical procedure ( Fig. 1 ).
Bioabsorbable Membranes
Bioabsorbable membranes have been developed primarily to avoid a second surgery for membrane removal. Various bioabsorbable materials, including polyglycoside synthetic polymers (ie, polymers of polylactic acid, polyglycolic acid, polylactate/polygalactate), collagen, and calcium sulfate have been frequently used in membrane barriers. Similar to ePTFE membranes, resorbable membranes are biocompatible and exert their function by excluding undesirable cells from migrating into periodontal defects and providing a space for ingrowth of periodontal attachment. The clinical efficacy of bioabsorbable membranes depends on their ability to retain their structural physical integrity during the first 6 to 8 weeks of healing and to be gradually absorbed thereafter. Based on this concept, chemicals and structural modifications (ie, polymerization, cross-linking) were incorporated into bioabsorbable membranes to extend their absorption time and increase the clinical effectiveness of these materials. However, the prolonged collagen resorption rate does not always result in greater periodontal regeneration. It is possible that membranes are only required to maintain their physical integrity for 6 weeks and prolonged retention after that is detrimental to the healing process.
Collagen barrier membranes, made primarily from bovine and porcine type I collagen, were successfully used in the treatment of periodontal defects. Collagen membranes are particularly suitable for GTR applications, as collagen is chemotactic and stimulates proliferation of fibroblasts, acts as a barrier for migrating epithelial cells, provides hemostasis, serves as a fibrillar scaffold for vascular and tissue ingrowth, can be easily shaped, and is readily adaptable. These membranes are resorbed by the enzymatic activity of macrophages and neutrophils.
Degradable polymers constitute a second major group of bioabsorbable barrier membranes. These membranes are formed by copolymerization of polylactic acid, polyglycolic acid, and polylactate/polygalactate. These synthetic membranes remain intact for 20 weeks or more, depending on their polymeric composition. Afterward they are degraded by hydrolysis of ester bonds and eliminated through metabolic pathways as carbon dioxide and water. Although these membranes are considered biocompatible, their hydrolysis is accompanied by a local inflammatory response. The inflammatory response is not considered harmful, but it remains to be established as to what extent it may affect the regeneration of periodontal tissues.
Calcium sulfate can act as an exclusion barrier and has been used as a GTR membrane in the treatment of periodontal defects. This material provides good adaptation to the margins of the periodontal defect and absorbs in about 30 days without triggering an inflammatory or foreign body reaction. Unfortunately, most of the data regarding the use of calcium sulfate in GTR come from either case reports or studies with a relatively small number of cases, in which calcium sulfate was used in association with bone grafting materials, preventing an adequate assessment of its clinical efficacy.
Numerous complications, such as early membrane degradation and epithelial downgrowth, were associated with the use of bioabsorbable barrier membranes. Nevertheless, a meta-analysis of intrabony and furcation defect studies investigating open flap debridement versus GTR revealed that most of the bioabsorbable and nonresorbable GTR membranes provide superior clinical results than open flap debridement alone ( Table 1 ). But only a few studies have directly compared the effectiveness of bioabsorbable membranes with open flap debridement as a control. Newer studies comparing the use of bioabsorbable membranes with nonresorbable ePTFE membranes indicate that both membranes are equally effective in the treatment of intrabony and furcation defects. The clinical indication of nonresorbable and bioabsorbable membranes necessitates consideration of the anatomy of the periodontal defect. Therefore, nonresorbable reinforced membranes and bioabsorbable membranes supported by filler materials are used for the treatment of nonsupportive defects, such as in wide 1- or 2-walled periodontal defects. On the other hand, narrower 2-walled defects can be treated with bioabsorbable membranes.
| Barrier | Intrabony Defects (mm) (Mean ± SD) | Furcation Defects (mm) (Mean ± SD) | |||
|---|---|---|---|---|---|
| CAL | PD | VPAL | VPD | HOPA | |
| Collagen | 0.95 ± 0.47 | 1.06 ± 0.37 | 0.1 ± 0.60 | −0.04 ± 0.52 | 0.96 ± 0.91 |
| Polymers | 0.92 ± 0.18 | 0.89 ± 0.14 | 2.5 ± 0.85 | 2.30 ± 0.74 | Insufficient data |
| ePTFE | 1.62 ± 0.25 | 1.41 ± 0.2 | 1.39 ± 0.36 | 1.01 ± 0.31 | 0.99 ± 0.31 |
Clinical outcomes
The clinical efficacy of GTR was reviewed exhaustively. Numerous randomized controlled clinical trials, case series, and case reports have shown that GTR is a successful reconstructive therapeutic option in the management of periodontal intrabony and furcation defects, albeit requiring adequate case selection and excellent surgical skills.
Treatment of Furcation Lesions
The clinical responses of furcation defects to GTR depend on their extent and location. Evidence indicates that GTR can be successfully used only in the treatment of class II mandibular furcations and has a limited clinical effect on class II maxillary furcations.
Class II mandibular furcations
Many randomized clinical trials have addressed the clinical outcomes of GTR in the treatment of class II mandibular furcations. A meta-analysis of these studies showed that GTR promotes superior clinical results than flap procedures alone in the treatment of class II mandibular furcations. GTR procedures resulted in significantly greater probing depth reduction when compared with open flap debridement alone (1.16 mm; 95% confidence interval [CI], 0.2–2.52; P <.001). Likewise, greater reductions in horizontal furcation depth were observed in sites treated with GTR, weighted mean difference 1.51 mm (95% CI, 0.39–2.62; P <.001). Application of barrier membranes consistently and predictably resulted in additional gains in horizontal clinical attachment compared with open flap debridement (1.73 mm; 95% CI, 0.61–2.85; P <.001). Although these results clearly indicate an advantage in the use of GTR for the treatment of class II mandibular furcations, complete furcation closure is a rare finding regardless of the treatment used. Additional gains in horizontal clinical attachment associated with GTR procedures allows for the transformation of class II into class I mandibular furcations, which are more easily maintained over time.
Class II maxillary furcations
The clinical effectiveness of GTR in the treatment of class II maxillary furcation defects was investigated in a few randomized controlled clinical trials. Meta-analysis of the reported clinical outcomes indicated only a limited added benefit from the placement of barrier membranes after elevation of a flap. For instance, class II maxillary furcation lesions treated with GTR failed to consistently show improved reduction in vertical probing depths when compared with sites treated with open flap debridement alone (mean difference, 1.42 mm; 95% CI, 0.28–2.55; P = .398). Also, GTR provided no additional gains in clinical attachment in class II maxillary furcations (0.76 mm; 95% CI, 0.29–1.22; P = .188). Although GTR promoted statistically greater reductions in the horizontal furcation depth compared with open flap debridement alone (1.05 mm; 95% CI, 0.46–1.64; P <.001), this difference has doubtful clinical significance and does not by itself support the indication of GTR for the treatment of class II maxillary furcations.
Class III furcations
Even though some clinical reports indicate that closure of mandibular class III furcations can be occasionally achieved with GTR, the efficacy of GTR in improving or eliminating class III furcations is unpredictable.
Treatment of Intrabony Defects
Data from most randomized controlled clinical trials indicate that treatment of intrabony defects with GTR results in significantly greater probing depth reductions and clinical attachment gains compared with open flap debridement alone. The observed differences in favor of GTR are supported by the results of a meta-analysis that shows that compared with open flap debridement, GTR results in an additional gain of 1.22 mm in clinical attachment level (95%CI, 0.80–1.64; P <.001) and further reduction of 2.21 mm in probing depth (95% CI, 0.53–1.88; P <.001). Clinical improvements associated with GTR are independent of the type of barrier membrane used (ie, nonresorbable, resorbable) and can be maintained over time.
The predictability of GTR in the treatment of intrabony defects was also addressed. Even though the variability in clinical outcomes obtained with GTR is high, the use of barrier membranes generally provides significant clinical advantages compared with open flap debridement in intrabony defects. A randomized controlled clinical trial showed that the percentage of intrabony defects showing gain in attachment levels was higher after GTR (50.9%) than after conventional flap procedures (33.3%).
Factors affecting GTR clinical outcomes
Regeneration of periodontal defects, although possible, is not always a predictable outcome. Several local and patient-related factors may account for the variability in the clinical responses to GTR. To increase the predictability and clinical success of GTR, factors related to the patient, the defect, and the surgical treatment should be evaluated during treatment planning.
Patient Factors Affecting Periodontal Regeneration with GTR
Many patient-related factors may adversely affect the healing outcomes after GTR procedures. Among these factors, smoking, poor plaque control, and residual periodontal disease actively receive special attention, as these can be controlled through behavioral and therapeutic interventions.
Smoking negatively affects the regenerative outcomes of GTR. Various mechanisms can contribute to the detrimental effects of smoking on healing after GTR, including decreased vascular flow, altered neutrophil function, decreased IgG production and lymphocyte proliferation, impaired fibroblast function, and increased prevalence of periodontal pathogens. The frequency and duration of smoking inversely correlate to clinical attachment gains after GTR. Moreover, a benefit of smoking cessation in patients undergoing GTR has been suggested. However, the time required for host responses to GTR to return to normal after smoking cessation has yet to be determined.
The level of postoperative plaque control and residual periodontal infection, evaluated by the number of residual periodontal pockets and percentage of sites with bleeding on probing, also affects the clinical responses to GTR. Barrier membranes are at a higher risk of becoming contaminated in individuals with high levels of periodontal pathogens and multiple sites with bleeding on probing. Therefore, patients should undergo GTR procedures only after periodontal infection has been treated. Ideally, patients should have full mouth plaque and bleeding scores equal to, or lower than, 15% to achieve optimal regenerative outcomes after GTR procedures.
Although there is not enough evidence that diabetes, immunosuppression, and stress impair the efficacy of GTR, it was reported that these patient-related systemic conditions could negatively interfere with the clinical outcomes of GTR.
Local Factors Affecting Periodontal Regeneration with GTR
The predictability of GTR in regenerating the periodontal apparatus is strongly influenced by the local anatomy and morphology of periodontal defects. Case selection is of paramount importance and represents 1 of the most significant factors in predicting the clinical outcomes of GTR procedures. The presence of cervical enamel projections and enamel pearls interferes with periodontal regeneration and should be removed during regenerative procedures. The gingival thickness around the affected area should also be analyzed, as gingival thickness less than 1 mm is associated with increased prevalence and severity of flap dehiscence over GTR membranes. Presurgical tooth mobility has a negative effect on the clinical outcome of GTR and should be controlled through splinting and/or occlusal adjustments. Local factors that favor plaque accumulation, such as calculus and overhanging restorations, need to be removed before GTR procedures. In addition to these considerations, specific factors related to the regeneration of furcation and intrabony defects should be evaluated.
Considerations regarding furcation lesions
The predictability and efficacy of GTR procedures in the treatment of furcation lesions are strongly influenced by the tooth anatomy, anatomic features of the furcation area, and morphology of the furcation defect. It was shown that the presence of root concavities could impair the results of GTR, as these concavities prevented adequate membrane adaptation. A lack of an intimate adaptation between the barrier membrane and the root surface allows for greater risk of apical migration of the junctional epithelium, thus obviating the barrier function of the membrane and subsequent regeneration. Therefore, when root concavities are present, the collars of barrier membranes should be modified to allow for improved membrane adaptation and clinical results. Another important parameter to be considered when evaluating a furcation lesion for GTR is the length of the root trunk. Molars with long root trunks (5–6 mm) show a higher frequency of clinical furcation closure after GTR therapy than molars with short root trunks (≤4 mm). The better prognosis associated with long root might be because coronal positioning of the flap, flap adaptation, and membrane coverage are better achieved in molars with long root trunks.
The clinical success of GTR in furcation defects is strongly affected by the defect morphology. In furcation defects, many aspects of the defect morphology are predictive of the clinical outcomes after GTR. Furcation defects with a horizontal depth of 4 mm or less and small distances between the furcation roof and (a) the base of the defect (4 mm or less), or (b) the crest of the bone (2 mm or less) are associated with improved clinical outcomes. Teeth with interproximal bone heights at the same level or coronal to the roof of the furcation, deep probing pocket depth, and narrow root divergence (3 mm or less) exhibit improved clinical outcomes and greater chances of complete furcation closure after GTR.
The regeneration potential of furcation lesions is also dependent on the location of the tooth in the mouth. Evidence indicates that although GTR can be successfully used in the treatment of class II mandibular furcations, it provides only limited advantages in the treatment of class II maxillary furcations. Differences in the clinical outcomes after GTR in maxillary and mandibular furcation defects is likely to be related to the anatomy of the defects, number of roots and furcations, access for root surface debridement, and membrane adaptation.
Considerations regarding intrabony defects
Clinical efficacy of GTR procedures in intrabony defects depends on the morphology of the defect. Clinical evidence indicates that after GTR, intrabony defects deeper than 3 mm show greater probing attachment gain and bone fill than shallow defects. However, the clinical outcome of GTR procedures in shallow and deep defects is similar when probing attachment gains are expressed as a percentage of the baseline intrabony component of the defects.
The width of the intrabony component of the defect, measured by the baseline radiographic angle formed between the bony wall of the defect and long axis of the root, plays an important role in determining the clinical outcomes of GTR. Therefore, narrow defects are consistently associated with increased amounts of probing attachment level gain and bone fill. A quantitative analysis of the effect of the width of the intrabony defects on the clinical outcomes of GTR revealed that intrabony defects with narrow radiographic angles (<25°) gained consistently more attachment than wide defects (>37°). Defect morphology in terms of the number of residual bony walls and tooth surfaces involved was reported to have minimal or no effect on the clinical outcomes of GTR. This is surprising, given that the potential for available PDL and mesenchymal cells may differ in different defect morphologies. It is possible that these studies have too low a power to detect differences in clinical outcomes. More studies are warranted to establish the effect of the number of residual bony walls on the clinical outcomes of GTR.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


