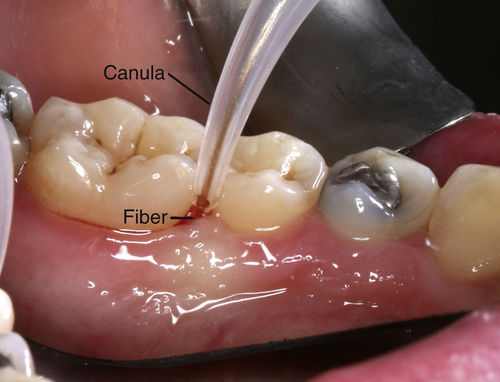
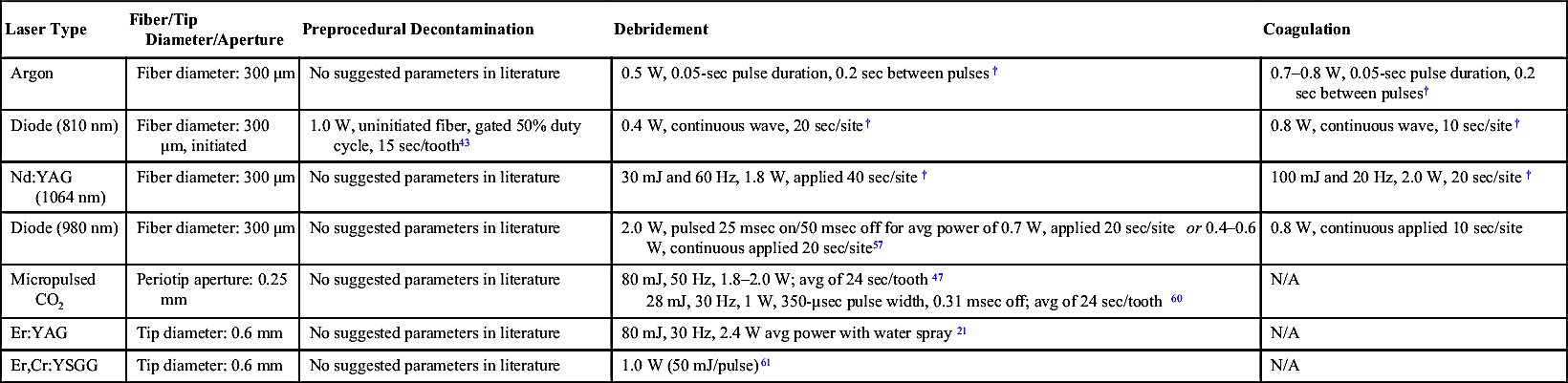
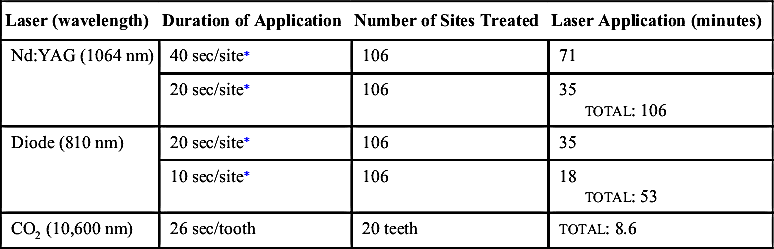
• Figure 3-1 Calibrating optimal laser fiber length using a periodontal probe.
When exiting the pocket, take care to inactivate the laser, preventing overexposure of the gingival margin’s thin tissue. If the fiber tip becomes irreversibly coated (Figure 3-4), cleave the fiber, calibrate the length of the fiber, and continue the procedure.
Completion of laser decontamination is determined by laser parameters used, delivery time, and clinical signs. Decontamination is accomplished with less mJ and more Hz than for coagulation. A more inflamed pocket may require less average power because of increased concentration of the laser’s preferred chromophores. With use of an initiated fiber and lower settings in continuous-wave mode, interaction is concentrated and will require less treatment time than with use of a noninitiated fiber with higher settings in pulsed mode. A deeper pocket will require longer treatment time because of increased surface area. As laser treatment progresses in an area, less and less debris should collect on the fiber. Fresh bleeding will occur, however, when the pocket wall is fully decontaminated and debrided (Figure 3-5). Keep in mind the laser parameters and application time. Observing tissue interaction is essential in determining the duration of laser exposure for the diseased site being treated.
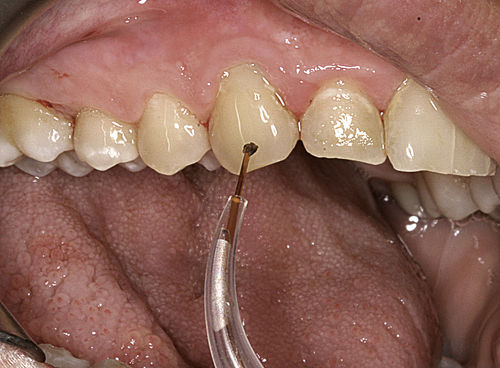
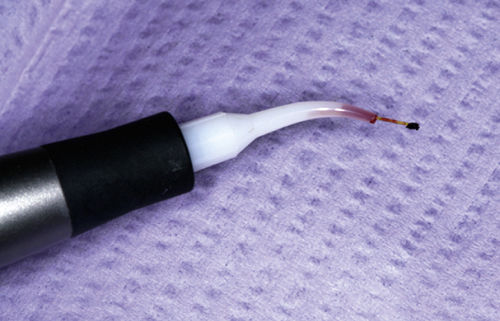
• Figure 3-4 Excessive amount of debris on fiber. At this stage of treatment, the fiber should be cleaved and recalibrated.
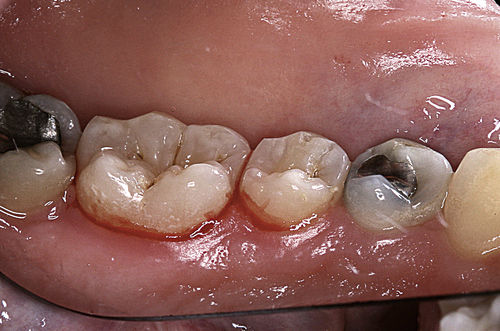
• Figure 3-5 Gingiva immediately after fiber-based laser treatment, showing fresh bleeding from the pocket.
Coagulation
When biofilm has been removed, the second objective in active phase I periodontal infection therapy is coagulation, sealing the capillaries and lymphatics of the healthy tissue. As previously noted, biofilm tends to continue its invasion of the host tissue through the vessels. Coagulation may inhibit the biofilm’s progression. It also counteracts the swelling that occurs with the inflammatory process. Coagulation is accomplished with increased mJ and decreased Hz compared with decontamination. Coagulation also requires less time within the pocket and does not address every millimeter of tissue.
For this procedure, a newly cleaved fiber is moved back and forth through the pocket, administering laser energy beyond the end of the fiber into the tissue. The application raises the temperature within the pocket slightly, to promote protein denaturation and sealing of the vessels. If continued hemorrhaging occurs on exiting the pocket, a freshly cleaved fiber may be used in noncontact mode to coagulate at the gingival margin, keeping the laser energy directed away from the tooth surface.
After coagulation, application of firm digital pressure to areas with deep pockets will support the re-adaptation of the tissue to the tooth and further enhance reattachment. Coagulation assists the first stages of healing after debridement.
Sulcular Debridement with Carbon Dioxide Laser
Whereas the argon, diode, and Nd:YAG lasers employ a contact technique for sulcular debridement, the micropulsed 10,600-nm CO2 laser uses a defocused, noncontact technique. Marginal dehydration and pocket decontamination are two steps applied in CO2 laser therapy. Because the CO2 laser’s wavelength is absorbed by the crevicular fluids and water content in the diseased tissue wall, it is important to direct the energy parallel to the tooth surface and toward the tissue.
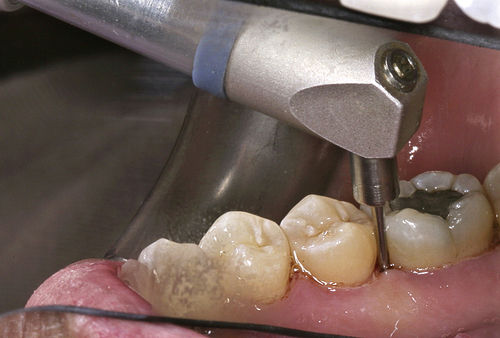
Begin the debridement procedure by directing laser energy to the coronal edge of the marginal gingiva. Hold the tip perpendicular to the tissue crest at a distance of approximately 1 mm. Tissue interaction is observed as a slight “frosting” of the surface (Figure 3-6). Marginal dehydration will improve entry of the tip by drawing the tissue slightly away from the tooth structure. Epithelial growth will be inhibited by this application. This is the first step before pocket decontamination.
The technique for decontamination involves placement of the laser’s defocusing tip 1 to 2 mm into the pocket (only 1 mm for pockets up to 6 mm in depth; only 2 mm for pockets greater than 6 mm in depth). Treatment should include the complete circumference of each tooth presenting with disease. Activate the laser as the tip is drawn through the crevicular space in an even, slow motion, working from the distal aspect to the mesial aspect on the buccal and again on the lingual side of the tooth. The laser tip is kept parallel to the long axis of the tooth (Figure 3-7).
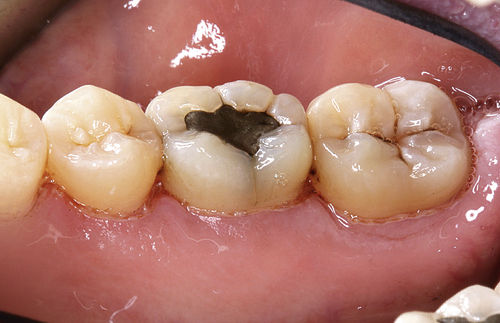
Treatment time is a maximum of 16 seconds per buccal or lingual surface. The length of application time depends on the extent of disease and the surface area; larger teeth such as molars are treated longer than smaller teeth such as lower anteriors.46 The tip must be kept open and free of coagulum for efficient energy flow. Keeping the tissue slightly moist and working in a single direction will enhance the laser’s efficiency. Vertical, up-and-down movements or pushing the tip toward the tissue can cause the tip to clog. If the tip becomes occluded, both the tip and detritus inside will continue absorbing the energy, with consequent excessive heating of the tip. Coagulation occurs simultaneously with decontamination. No additional steps are necessary in using the CO2 laser. Figure 3-8 shows the gingiva after laser treatment.
• Figure 3-6 Laser marginal dehydration of gingival tissue immediately before pocket debridement and decontamination with CO2 laser.
Postoperative Care
The therapeutic appointment concludes with several steps in professional postoperative care. After the laser treatment, allow the patient to rinse with water or with a non–alcohol-based rinse to freshen and moisten the mouth. A topical soothing agent such as vitamin E oil or aloe vera may be applied with a gloved finger or sterile cotton swab to the areas treated. Firm adaptation of tissue to the tooth with digital pressure may assist adhesion of fibrin between the tissue and tooth, particularly for deeper pockets.
Postlaser irrigation is a subject of debate. Although irrigation with chlorhexidine or other solutions is used in conventional treatment as a final step in disinfecting periodontal pockets, the authors believe that postlaser irrigation is unnecessary. In fact, Mariotti and Rumpf47 found that solutions of chlorhexidine (0.12% or less) in contact with wound sites for even a short time could have serious toxic effects on gingival fibroblasts. Other studies report that subgingival irrigation has no significant additive effects on periodontal healing.48–50
When the laser procedure is completed, all the benefits of profound decontamination and coagulation are in place. Further manipulation of the tissues will reintroduce contaminated instruments into the pocket and disrupt the fibrin clot.
The final step in postoperative care is advising the patient on what to expect, addressing further concerns, and discussing continued self-care. Counsel the patient that mild discomfort is possible the first 24 to 48 hours. With laser-assisted nonsurgical periodontal therapy, discomfort is associated more often with root debridement than with the laser treatment. Excessive pain may indicate another issue and warrants investigation. The patient should avoid spicy, sharp, and crunchy foods for 24 hours to help prevent discomfort and trauma. Seeds and husks may become lodged between the gum and the tooth and should be avoided. The risk of impaired healing from presence of a foreign object is highest in the first few postoperative days, but risk may persist if the periodontal disease is more severe. Encourage the patient to be diligent in supporting the healing process by consistent and thorough daily cleaning. Patients appreciate instructions in written form for reference as well as in verbal form (Box 3-1).
Healing and Tissue Rehabilitation
Healing typically occurs without complications as the body responds to laser therapy. Through the first 24 to 72 hours, the patient may feel tenderness and experience light bleeding when eating or cleaning; after tooth debridement and tissue decontamination, epithelium begins to regenerate after 24 hours and then progresses 1 mm per day, protecting the pocket wall. Within 1 week, the wound surface is covered. Connective tissue begins proliferation by day 5. Because laser decontamination procedures are repeated at 10-day intervals, the epithelium is impaired during laser biofilm reduction. This allows the fibroblasts to continue organizing into a connective tissue attachment apparatus. The attachment will continue to mature for 12 months.8 This attachment is easily disrupted, so only light-pressure probing is recommended after several months, with resumption of normal probing after 6 months of postoperative therapy.51
Classic signs of tissue rehabilitation include improved color, consistency, texture and stippling, and contour. These signs, as well as a decrease in or elimination of hemorrhaging during gentle tissue manipulation and a reduction of pocket depth, all are desired indications of tissue healing. Figure 3-9 shows initial periodontal probing before treatment and periodontal probing 6 months after treatment. Figure 3-10 shows data for three sets of probings: initial probing, 8 weeks after treatment, and 5 months after treatment. Analysis of the probing results shows a resolution of 86% of the bleeding sites, a decrease in 86% of the pocketing sites, and a 58% decrease in the number of teeth exhibiting periodontal disease.
Complications and Adverse Reactions
Healing may be complicated by microbiologic, immunologic, and traumatic factors. Rapid pocket reinfection will occur with insufficient removal of subgingival biofilm, with inadequate supragingival biofilm control, or with uncorrected poor restorative conditions. Patients with a compromised immune system often exhibit a delayed or less optimal healing response; with the assistance of laser benefits, however, such patients could recover better than expected.
Traumatic injury, such as from excessive instrumentation or increased collateral damage from overexposure of laser energy, may result in prolonged tissue discomfort and soreness. Systemic diseases such as diabetes are associated with delayed healing. Evaluation for excessive occlusal stress, which may impair healing, also is important.
In states in which hygienists are allowed to perform laser therapy, it is incumbent on the hygienist to make certain that the dentist has performed a complete occlusal evaluation and correction/equilibration of the dentition. Occlusion is a risk factor for periodontal breakdown, so occlusal treatment needs to be considered as a part of the comprehensive treatment of periodontal disease.52 Trauma from occlusal problems constitutes an additional risk factor for the progression and severity of periodontal disease. An understanding of the effect of trauma from occlusal loading on the periodontium is useful in the clinical management of periodontal problems.53
Adverse reactions are a result of inappropriate laser application: The wavelength is incorrect for the target site, the parameters are incorrect, or the duration of application is incorrect. Some lasers interact with metal, resulting in a percussive reaction and immediate heat generation. This heat could transfer to the nerve or surrounding tissue. Therefore the laser energy must not be directed toward metallic crown margins or metallic restorations at the gingival margins. Laser energy directed toward the tooth may cause irreversible damage to the tooth, such as cracking, pitting, melting, or charring. Excessive heat accumulation can cause damage to underlying bone. Intense overexposure of laser energy on tissue results in carbonized tissue.
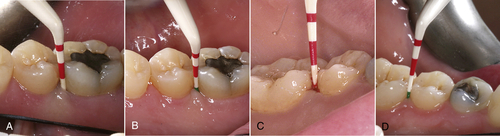
• Figure 3-9 A, Periodontal pocket probing before CO2 laser treatment. B, Periodontal pocket probing 6 months after CO2 laser treatment. C, Periodontal pocket probing before fiber-based laser treatment. D, Periodontal pocket probing 6 months after fiber-based laser treatment.
Complications in healing and adverse reactions are minimized with adherence to appropriate treatment protocols. Understanding the laser settings and observing the laser–tissue interaction during application are crucial. The clinician must have adequate knowledge and proficiency whenever providing treatment with any instrument, whether ultrasonic, hand, or laser. All of these treatment modes are extremely beneficial when used properly.
Documentation
Documentation in the patient’s chart serves to describe the conditions, diagnosis, and specific treatment rendered. Written references concerning laser use should include the following:
• The wavelength and type of laser (e.g., 980-nm diode)
• The spot size (size of fiber, tip, or aperture of tip)
• The settings (e.g., mJ, Hz, W of average power)
• Mode of application (continuous or pulsed wave)
• Duration of application
• Application with or without water spray
• Type of anesthesia used (topical or injection, number of carpules)
Documentation also should (1) confirm that wavelength-specific laser safety glasses were worn, (2) describe any adverse reactions and how they were managed, and (3) state that postoperative instructions were given. Complete documentation should include content that serves to answer any question that a patient might ask about the treatment (Box 3-2).
Adjunctive Chemotherapeutics
The rationale for adding antibiotics in some cases is better biofilm suppression with concomitant therapies. If treatment requires multiple strategies, chemotherapeutics may be used in conjunction with lasers and conventional treatment. Antibiotics such as doxycycline, tetracycline, and metronidazole are prescribed for systemic control of pathogens. Low-dose levels of oral doxycycline hyclate (Periostat) suppress the enzymatic activity of collagenase associated with the disease processes. Topical preparations of minocycline hydrochloride (Arestin), doxycycline hycylate (Atridox), and chlorhexidine gluconate (as the Periochip) may be placed to control localized growth of pathogens.
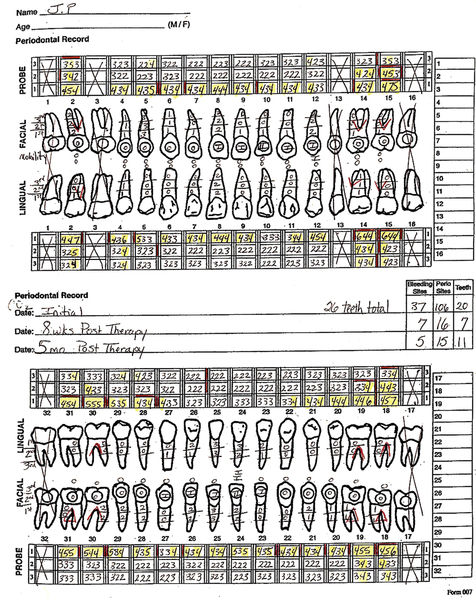
• Figure 3-10 Periodontal charting showing pocket depths initially and at 8 weeks and then 5 months after laser treatment.
Biofilm is most susceptible to chemotherapeutics when it has been disrupted aggressively through debridement of both the tooth and the tissue. A systemic antibiotic should begin early in the treatment to assist the body as treatment progresses. A local antibiotic may be placed in an affected site after the last session of laser treatment; in this way, without disturbance, the effectiveness of the drug is maximized, because it works for several weeks. Locally administered antibiotics should not be used between lasing appointments.
It is prudent to assess the biofilm’s composition and the susceptibility of pathogens before administration of chemotherapeutics. Many types of culturing do not test the antibiotic against the protective mechanisms in the biofilm and thus do not reflect the in vivo environment. Polymerase chain reaction (PCR) assay, 454 sequencing, and other molecular tests give a better view of what organisms are cohabitating within the biofilm. This information may determine which medication(s) would be most beneficial, how long it should be administered, and whether a second phase with testing is needed. Chronic wounds should never be controlled merely with antibiotic therapy.
Laser Safety
The designated laser safety officer is responsible for educating the dental team in the safe use of the laser, as well as for enforcing safety practices, as follows:
1. Securing the operatory by limiting access to the room and posting “laser in use” signs
2. Using safety features of the laser, such as placing the laser in standby mode when not in use
3. Enforcing mandatory use of wavelength-specific protective eyewear within the treatment area
4. Evacuating with high-volume evacuation to remove aerosols and laser plume
5. Using a high-efficiency particulate filtration mask (particle filtration efficiency of 99.75% at 0.1 μm)
Test firing the laser before the patient’s procedure is another important step in safety and procedure preparation. Test firing proves the laser energy is being delivered as expected. For this procedure, with safety measures in place, position the terminal end of the laser away from the patient. Select a suitable chromophore (for argon, Nd:YAG, and diode lasers: dark material; for CO2 lasers: moist paper; and for erbium lasers: water) and activate the laser while holding it 1 to 2 mm from the material chosen. As energy is absorbed, an interaction will be observed, such as a mark and plume or water bubbling or evaporating. This test step is not the same as “initiating” the fiber but simply constitutes an assessment of interaction between the laser and an appropriate chromophore, to ensure the laser is working as anticipated.
Laser Plume
Although no set standard exists, strong recommendations (such as those from the Occupational Safety and Health Administration [OSHA], the Centers for Disease Control and Prevention [CDC], and the American National Standards Institute [ANSI]) address evacuation of the laser plume. High-volume evacuation is indicated for aerosol reduction during ultrasonic instrumentation,54 as well as for plume removal during laser treatment. The plume is composed of 95% water and 5% particulate matter, organic and inorganic chemicals, and microorganisms.55 Organic chemicals such as benzene, toluene, formaldehyde, and cyanide have been isolated within the plume; inorganic chemicals include carbon monoxide, sulfur, and nitrogen compounds.56 The microorganism analysis shows bacteria, microbacteria, fungi, viruses, and DNA from intact viruses of human immunodeficiency virus (HIV), hepatitis B virus (HBV), and human papillomavirus (HPV).57 Most particles are 0.3 to 0.5 μm in size, 90% of which are likely to be inhaled and deposited on the alveolar lung tissue.58 Therefore the common mask that filters only 5.0-μm particles provides inadequate filtration. Use of a mask filtering 0.1-μm particles is recommended.55
The combination of high-volume evacuation and a high-efficiency filtration mask decreases the exposure risk associated with ultrasonic and laser procedures. These masks are available in tie-on and ear-loop styles through dental supply companies.
Technical Aspects of Laser Settings
The treatment objective, fiber size, and existing chromophore concentration should be considered in choosing the settings for a nonsurgical periodontal procedure. For laser-assisted hygiene applications, lower settings are required for thorough decontamination of the tissues. The fiber size can directly affect the amount of energy the target receives with a specific setting. A 320-μm fiber has a smaller spot size, which increases the power density at the target, compared with a 400-μm fiber. With the same setting, a 400-μm fiber delivers only 64% of the power density delivered with a 320-μm fiber.
The concentration of chromophore present in the target tissue also will affect settings. Diseased tissue has an increased amount of hemoglobin and responds well to certain wavelengths, requiring less energy. Fibrotic tissue, with decreased vascularization and hemoglobin, comparatively needs more energy.
Printed parameters from laser manufacturers are only guidelines for treatment. Observed tissue interaction is the key to knowing if the settings are adequate or need adjustment. A rule of thumb is to use the minimum amount of energy to achieve the therapy needed. Reference sources for settings have been added, for convenience, to the suggested parameters in Table 3-1.59–61 Although each of the listed lasers may be used in nonsurgical periodontal procedures, some are more efficient than others. One laser used for therapy may require more or less treatment time than another, which affects treatment planning. Table 3-2 contrasts the time investment required with three types of lasers. The patient undergoing treatment exhibited 87 “bleeding on probing” sites, 106 sites of 4-mm depth or greater, and 20 involved teeth. Because of treatment protocol, the Nd:YAG and diode lasers reflect treatment of each site exhibiting disease, whereas the CO2 laser reflects treatment per tooth.
Fiber
The fibers used with argon, diode, and Nd:YAG lasers are constructed similarly and are manufactured in a variety of diameters, with the 300- to 400-μm fiber most often used for laser-assisted hygiene procedures. The fiber has four parts: the jacket, cladding, fiber, and coupler. The jacket is a thick, flexible, clear or translucent, latex-like covering, or in some models, a thin, tougher plastic, that protects the fiber. The cladding is a coating on the outside of the fiber that is inwardly reflective, collimating the laser beam completely to the fiber’s terminal end. The fiber itself is made of quartz and is crystalline in structure. The coupler connects the fiber to the laser.
Proper handling of these parts is critical for optimal power delivery. The extra length of fiber should be loosely coiled and secured away from rolling chairs or sources of entanglement. Manufacturers produce accessories to help manage the extra length of fiber. When preparing the fiber for the handpiece, strip away as little of the jacket as possible. The fiber should be bare through the cannula but not at the point at which the collet nut in the handpiece tightens. Damage to the cladding can occur with use of an improperly sized stripping tool. Overclosing on the fiber during stripping will nick the cladding. Should the cladding become scratched, laser energy will be lost from that site, decreasing the power at the working end. Inspect the fiber for light leaks of the aiming beam before inserting it into the handpiece. If light leaks are present, strip the fiber farther back, and cleave the fiber just behind the point where visible light is detected.
Fiber breakage may occur if the fiber is coiled too tightly or retracted repeatedly in a cassette. Breakage may also occur while working in the pocket. If a metal cannula is used, the side of the fiber may rub against the cannula’s edge, inadvertently scoring the fiber and leading to breakage. If such breakage goes undetected, unwanted exposure to laser energy may result. Loss of fiber integrity can waste valuable treatment time, diminish power needed for treatment, and constitute a hazard.
Initiating the Fiber
Initiating the fiber is helpful with some laser-assisted hygiene procedures but is not desired in others. Initiation of the fiber tip is accomplished by activating the laser while touching the fiber to a dark chromophore. Though many practitioners use articulating fiber or cork, neither produces a satisfactory, complete or thorough initiation. The best initiation is performed by using black ink suitable for painting onto glass surfaces (available at any art supply or hobby store) and a good quality, very thin paintbrush. The paintbrush is dipped into the ink and painted onto the tip of the laser fiber and allowed to dry for 30 seconds. The purpose is to concentrate heat energy at the fiber’s tip, increasing the thermal interaction with the tissue and accelerating debridement.
Initiation is used with lasers of lower fluence, particularly diode lasers, in the decontamination procedure. Because an initiated fiber concentrates the laser energy at the point of tissue contact, heat can accumulate within the tissues quickly. Application time should be limited to minimize collateral damage in surrounding tissue. Lower settings are used in continuous-wave mode for a shorter duration to accomplish decontamination of the pocket wall. Also, in working with fibrotic tissue exhibiting less chromophoric concentration, initiation is helpful.
If the objective is penetration of the laser energy into the tissue beyond the fiber, the fiber is not initiated. An uninitiated fiber is used for preprocedural decontamination and coagulation. The Nd:YAG, a free-running pulsed laser, does not require initiating because of its high peak powers and immediate interaction with the tissue. Argon and diode lasers may be used in pulsed or continuous-wave mode, with an uninitiated fiber used for preprocedural decontamination and coagulation. Continuous-wave mode requires less energy and shorter application time, which will minimize heat accumulation within the tissue. The pulsed-wave mode may use higher settings with slightly longer treatment times. The off time between pulses allows heat dissipation within the tissue.
The clinician must have a clear understanding of the laser effects with an initiated or uninitiated fiber and must be proficient in reading tissue–laser interaction.
Cleaving the Fiber
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses