Fig. 18.1
EDTA promotes SCAP survival. Organotype immature teeth root canal models were irrigated with 20 ml of irrigant for 1 min followed by thorough rinsing with Hanks’ Balanced Salt Solution for 7 days. SCAPs were then seeded with platelet-rich plasma (PRP) scaffold into the root segments. The percentage of viable cells (vimentin positive) of total cells (TO-PRO-3 positive) were determined by confocal microscopy for each group after 21 days. 17 % EDTA group demonstrated the maximum number of viable cells followed by EDTA/NaOCl. No viable cells were seen for the EDTA/CHX and NaOCl/EDTA/NaOCl/isopropyl alcohol (IPA)/CHX groups (Modified from Trevino et al. [22]). *** P < .001
Another ex vivo study by Galler et al. [23] evaluated the effects of full-strength (5.25 %) NaOCl compared to 17 % EDTA on dentin surface. Dentin cylinders used as cell carriers were subjected either to 5.25 % NaOCl or 17 % EDTA. Dental pulp stem cells (DPSCs) with biodegradable hydrogel scaffold enhanced with bioactive molecules such as heparin-binding growth factors vascular endothelial growth factor (VEGF), transforming growth factor-beta1 (TGF-β1), and fibroblast growth factor-2 (FGF-2) were loaded into the cylinders which were in turn implanted into immunodeficient mice. The histological results of the study clearly demonstrated that dentin treated with 5.25 % NaOCl leads to resorption and clastic cellular activity along the dentinal walls. On the other hand, dentin conditioned with 17 % EDTA promoted the formation of pulp-like tissue with blood vessels and polarized cells that often extended processes into dentinal tubules and expressed the odontoblastic marker dentin sialoprotein (DSP) (Fig. 18.2). One study evaluated the effects of 5.25 % NaOCl or 17 % EDTA dentin conditioning on stem cell expression of odontoblastic markers [24]. Dentin disks were treated (conditioned) with 5.25 % NaOCl or 17 % EDTA. The expression of odontoblastic markers such as matrix extracellular phosphoglycoprotein (MEPE), dentin matrix protein-1 (DMP-1), and dentin sialophosphoprotein (DSPP) was evaluated using quantitative reverse-transcription polymerase chain reaction (qRT-PCR). This study demonstrated that tooth slices subjected to 5.25 % NaOCl showed no expression of the abovementioned markers. However, the ones treated with 17 % EDTA showed significant increase in these markers in vitro (Fig. 18.3). Moreover, when tooth slices were implanted into the dorsum of immunodeficient mice, similar results were obtained at 14 and 28 days. It is also noteworthy that this prolonged effect of dentin conditioning with NaOCl was detected long after the irrigant had been removed, suggesting that NaOCl has a profound indirect effect on stem cell toxicity [25]. Thus, dentin conditioning with sodium hypochlorite at its maximum used clinical concentration leads to greatly diminished stem cell survival and loss of odontoblast-like cell differentiation.
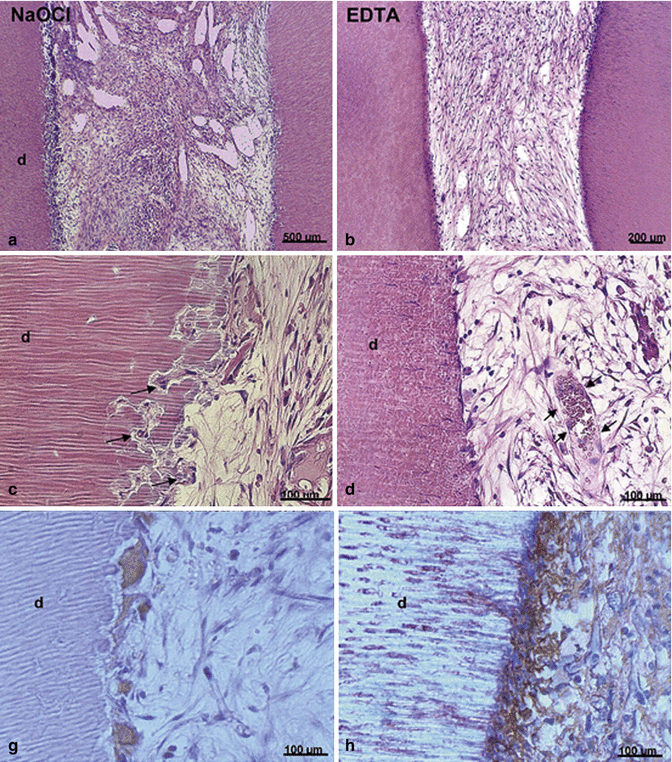
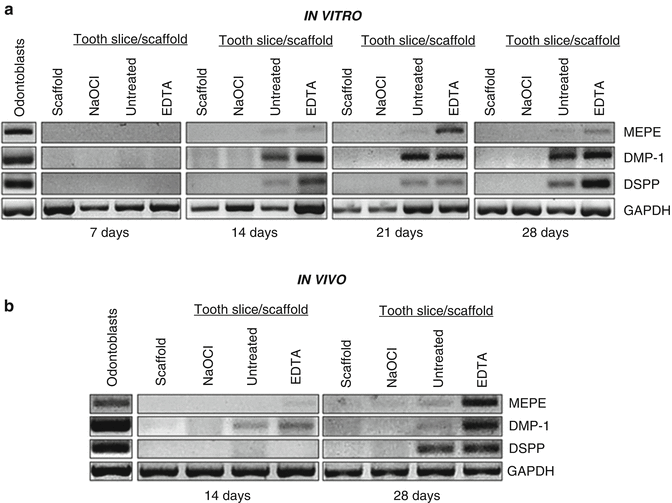

Fig. 18.2
EDTA promotes pulp-like tissue formation and DSP expression. Dentin cylinders were irrigated with 5.25 % NaOCl or 17 % EDTA. DPSCs mixed with hydrogel scaffold were loaded into the cylinders. Dentin cylinders were then implanted into immunodeficient mice. Hematoxylin and eosin staining and tartrate-resistant acid phosphatase (TRAP) done at 6 weeks show the presence of disorganized fibrous connect tissue and presence of large multinucleated giant cells/odontoclasts in the NaOCl group (panels a, c, g) in the NaOCl group. Well-organized vascularized connective tissue with cells at the cell-dentin interface that appear flat and are in close contact with the dentin wall (panels b, d). Immunohistochemistry for DSP demonstrates that cells adjacent to the dentin surface stain positive for DSP, which indicates that these cells have differentiated into an odontoblast-like phenotype (panel h) (Modified from Galler et al. [23])

Fig. 18.3
EDTA promotes odontoblastic differentiation of stem cells. Scaffold without tooth slice was used as a negative control. Tooth slices were treated with 5.25 % NaOCl for 5 days (to denature dentin proteins), left untreated, or treated with 17 % EDTA for 1 min (to mobilize dentin proteins). Markers of odontoblastic differentiation, i.e., DSPP, DMP-1, and MEPE, were evaluated by RT-PCR. For in vivo studies, tooth slices were treated with the same irrigation protocol and were then loaded with scaffold and SHED cells. They were then implanted subcutaneously into the dorsum of immunodeficient mice. After 14 or 28 days, markers of odontoblastic differentiation (i.e., DSPP, DMP-1, and MEPE) were evaluated by RT-PCR. Both studies demonstrated increased expression of all markers at in the untreated and 17 % EDTA groups whereas no expression was observed in the scaffold only and 5.25 % NaOCL groups (panels a, b) (Modified from Casagrande et al. [24])
Another recent study was conducted to evaluate whether other clinically used concentrations of NaOCl were conducive for stem cell (SCAP) survival and differentiation [26]. Standardized root canals were prepared in extracted human teeth. The prepared teeth were then irrigated with NaOCl at the concentrations of 6, 3, and 1.5 %. Approximately half of the samples received a second irrigation with 17 % EDTA, whereas all samples received a copious final flush with saline to remove any residual chemical from the canal space. SCAP in a hyaluronic acid hydrogel were seeded in all canals and cultured for 7 days. The number of viable cells was assessed using a luminescence assay, while the level of DSPP was assessed by real-time RT-PCR. It was found that dentin conditioning with NaOCl decreases both SCAP survival and differentiation in a concentration-dependent manner. However, the concentration of 1.5 % of NaOCl was found to have minimal effects on the survival and differentiation. In addition, it was demonstrated that a final irrigation with 17 % EDTA reverses the deleterious effects of NaOCl (Fig. 18.4). Thus, this study agrees with other studies that dentin conditioning with 6 % NaOCl has a negative effect, while 17 % EDTA has a positive effect on the survival and differentiation of stem cells subsequently cultured in contact with the conditioned dentin [22, 26, 27]. The negative effects of NaOCl do not appear to be directly related to residual NaOCl in the dentinal tubules resulting in direct toxicity since neutralization with sodium thiosulfate (5 %) did not reverse this effect [26]. Thus, NaOCl has a profound effect on dentin resulting in diminished stem cell survival and differentiation. These effects can be minimized by using 1.5 % NaOCl followed by 17 % EDTA [19]. Collectively, all studies mentioned here point to the detrimental effects of full-strength NaOCl and the beneficial effects of 17 % EDTA on dentin.
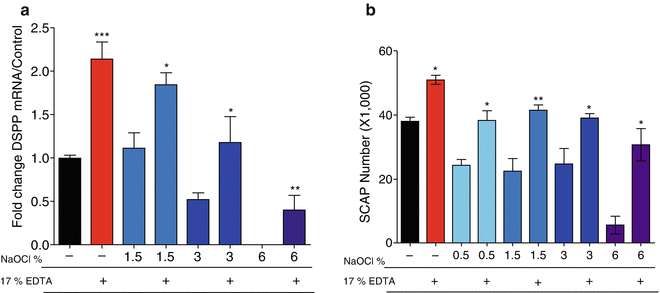

Fig. 18.4
Sodium hypochlorite decreases SCAP survival and differentiation in a concentration-dependent manner. Organotype immature teeth root canal models were irrigated with different concentrations of NaOCl following a standardized protocol that included a final wash of saline or EDTA. SCAPs were seeded into the root segments and cultured in vitro for 7 days. The percentage of viable cells were determined by a luminescence assay. NaOCl concentration-dependent decrease in SCAP survival is partially reversed by a final irrigation with 17 % EDTA (panel a). In addition, real-time qRT-PCR was used to determine the expression of the odontoblast-like cell marker dentin sialophosphoprotein (DSPP) mRNA. NaOCl decreases DSPP expression in a concentration-dependent manner with no expression seen in the group treated with 6 %. In addition, EDTA partially reversed the negative effect of NaOCl on DSPP expression (panel b). Data are presented by % of maximum observed effect on the EDTA only-treated group (control) (Modified from Martin et al. 2014 [26])
Irrigants and Dentin Matrix Growth Factors
Important biologically active growth factors are trapped in the dentin matrix during dentinogenesis. Some of these growth factors such as VEGF [28] and TGFB1 [29] are known to have a robust effect on the differentiation and/or proliferation of mesenchymal stem cells. These growth factors appear particularly efficacious in promoting the proliferation of mesenchymal stem cells and directing them toward an odontoblast-like phenotype [30, 31]. Irrigants, especially NaOCl in high concentration, are known to denature these dentin-derived growth factors [32]. In an in vivo study, dental pulp stem cells (DPSCs) proliferated at higher rates and expressed higher levels of odontoblastic markers in a tooth slice model compared to DPSCs placed in scaffold only [27]. These findings suggest that morphogens, such as the many growth factors known to be present in dentin, are sufficient to promote the survival, proliferation, and importantly the differentiation of dental stem cells. EDTA is known to solubilize and mobilize these growth factors from dentin, thereby increasing their bioavailability [33, 34]. Thus, its use may allow clinicians to harness the inductive properties of dentin-derived morphogens and growth factors normally present in dentin [35]. Therefore, the indirect negative effect of NaOCl and positive effect of EDTA on stem cell proliferation and differentiation appear to be directly related to the denaturing and solubilizing effects of these irrigants, respectively, on dentin matrix proteins. Astute clinicians must use the best available evidence to choose the combinations and concentrations of irrigants to achieve the greatest antimicrobial effect while minimizing stem cells death and loss of differentiation potential.
Stem cell survival, proliferation, and differentiation are also known to be dictated by the surface on which the cells grow [36–38]. Stem cells attach to a specific surface such as a target organ during organogenesis, or repair, via the interaction of specific cell-adhesion molecules such as integrins expressed on the plasma membrane of these cells. The effect of the substrate on stem cell behavior is best illustrated by the effect of the stem cell niche that in addition to growth factors (discussed above) provide attachment signals resulting in cell arrestment in a quiescent state [39, 40]. Cells released from their niche become “activated” and start proliferating and undergoing differentiation. The process of culturing tooth-derived stem cells such as DPSCs or SCAP is a good example of cells leaving their inhibited state in the niche (dental pulp or apical papilla, respectively) and displaying remarkable proliferative and differentiation potentials. This information has strong clinical implications since the dentin matrix composition (stem cell substrate) is altered by chemical treatment during the process of chemical debridement. NaOCl is known to cause changes in dentin matrix composition with decrease in carbon and nitrogen content and demineralization when used at high concentrations [41]. In contrast, the concentration of 1 % NaOCl does not cause any significant changes in dentin composition or mechanical properties. The property of attachment to a substrate has been evaluated using various other irrigants as well [42]. Ten treatment groups with different combinations of irrigants were used to evaluate attachment of stem cells from human exfoliated deciduous teeth (SHED) cells to dentin walls. It was found that groups treated with NaOCl and chlorhexidine (CHX) appeared to have the lowest amount of cell attachment compared to the groups that were treated with Aquatine EC and Morinda citrofolia (MCJ) (Fig. 18.5). An interesting finding is that all groups that were treated with EDTA as a chelating agent showed maximum number of cell attachment than groups that were treated with either no chelating agent or MTAD (Fig. 18.5). Thus, changes in dentin’s chemical composition could interfere with the ability of stem cells to attach, proliferate, and differentiate on the dentin surface. Thus, NaOCl is likely to affect stem cell fate by altering the dentin’s chemical composition, including the denaturation or growth factors and attachment molecules.
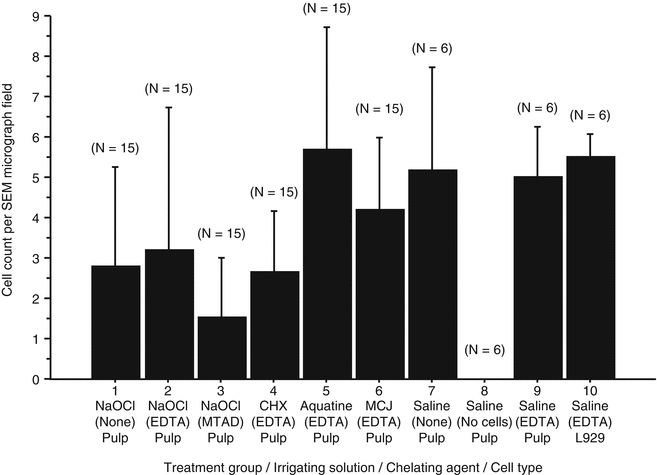

Fig. 18.5
EDTA promotes cell attachment to dentin. Tooth segments were treated with various irrigants during instrumentation followed by the use of a chelating agent and a final rinse with the first irrigant. SHED cells were then loaded into the segments, and after 7 days, the number of cells (L929 and SHED) attached to the root canal walls per SEM micrograph field of view was assessed. Rat fibroblast L929 cells were used as positive control. The rank order of cleaning and shaping treatments from the lowest to the highest mean numbers of attached DPSCs was NaOCl/MTAD, CHX/EDTA, NaOCl, NaOCl/EDTA, MCJ/EDTA, and AquatineEC/EDTA (Modified from Ring et al. [42])
Irrigation Techniques
Apart from the choice of chemical irrigant, focus may also be given to the types of techniques used to irrigate these teeth. Apical negative-pressure irrigation has been advocated for its superior disinfection and safety properties [43, 44]. Recent studies comparing bacterial counts in immature dog teeth after use of EndoVac versus conventional positive-pressure irrigation along with triple antibiotic paste (equal mixture of minocycline, metronidazole, and ciprofloxacin (TAP)) failed to demonstrate significant difference in bacterial reduction in the EndoVac group (88.6 %) versus conventional irrigation (78.28 %) [45]. However, the qualitative histological evaluation of the tissues formed following the regenerative/revascularization procedure in both groups suggests that the EndoVac irrigation promoted better formation of connective tissue, blood vessels, and mineralized masses while displaying lesser inflammatory cells in the EndoVac group than in the conventional irrigation group [46]. Moreover, the periapical region showed the presence of osteoclasts and bone resorption. These findings could be due to inadequate disinfection as well as any extrusion of NaOCl that may have impaired pulpal and periapical healing/repair [46]. It is important to emphasize that more studies evaluating the effect of different chemical debridement approaches such as sonic, ultrasonic, and negative pressure on regenerative outcomes are needed. Ideally, these studies should have quantitative outcomes and appropriate sample size to allow for more robust evidence in this important subject. Collectively, additional studies evaluating the effects of other irrigation techniques are warranted to fully optimize the irrigation protocol.
Residual Intracanal Medicaments and Stem Cell Survival
Regenerative procedures are typically performed in multiple visits with placement of an intracanal medicament to maximize disinfection and successful outcomes. Most of the published case reports and case series have utilized either the TAP or calcium hydroxide as inter-appointment medicaments [12]. Although the antimicrobial effect of these agents has been widely appreciated [47–49], their effect on stem cell survival was largely unknown until recently. A study sought to evaluate the direct effect of different medicaments on SCAP survival [50]. It was found that antibiotic paste formulations at the concentration typically used in previously published cases were directly lethal to SCAP. Interestingly, calcium hydroxide had no detrimental effect; instead it promoted survival and proliferation [50]. Another study evaluated the residual effect of calcium hydroxide or TAP on the survival of SCAP [51]. In this study, dentin disks were exposed to TAP or calcium hydroxide for 7 or 28 days, followed by irrigation with 1.5 % NaOCl and 17 % EDTA to remove the medicament. Similar to the direct effect, the TAP at the paste-like consistency had a detrimental residual effect, greatly impacting stem cell survival on the conditioned dentin [51]. Conversely, dentin conditioning with calcium hydroxide promoted survival and proliferation. Therefore, the adequate removal of intracanal medicaments, in particular antibiotic formulations, appears to be a challenging step following irrigation of the root canal system prior to the delivery of stem cells.
A study was conducted to evaluate the effectiveness of different irrigation methods to removal of triple antibiotic medication from the root canal system (canal lumen and dentinal tubules) [52]. Greater than 85 % of the medicament was found remaining within the dentinal walls despite the use of ultrasonic and sonic activation and negative-pressure (EndoVac) and conventional positive-pressure irrigation (Max-i-Probe needle). These findings were surprising and have profound clinical significance. Extensive penetration of TAP was observed as seen by direct visualization of staining often extending to the cementum layer in dentin disks treated with the medication [52]. Although high penetration into dentin appears to be a desirable effect for an antimicrobial agent, its negative effect on stem cell survival must be taken in clinical consideration. Importantly, it has been previously demonstrated that if used at the concentration of 1 mg/ml, it has minimal effect on the survival of stem cells [51]. Thus, the undesirable effects of the triple antibiotic medication can be greatly minimized with the use of a concentration that retains its adequate antimicrobial effect [48] but has minimal residual effect on the survival of stem cells [51]. Nonetheless, irrigation techniques must be optimized to allow better removal of medicaments with possible deleterious effect on stem cell fate and the exposure of attachment molecules and growth factors that maximize the survival, proliferation, and odontoblastic differentiation along the dentinal walls.
Overview of a Regenerative Endodontic Procedure
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


