Introduction
Our objective was to evaluate the influence of saliva contamination on the shear bond strength of orthodontic brackets bonded with self-etching primers.
Methods
One hundred thirty-five bovine incisors were randomly divided into 3 groups, and exposed enamel surfaces were bonded with Transbond Plus Self Etching Primer (TB) (3M Unitek, Monrovia, Calif), Adhese Single Bottle (AD) (Ivoclar, Vivadent, Schaan, Liechtenstein), and Self Etch Bond (SE) (Vigodent, Rio de Janeiro, Brazil). Each group was subdivided into 3 treatments (n = 15): saliva contamination (S), saliva contamination and deionized water rinsing (SW), and no salivary contamination (C). Resin composite (Z-100, 3M/ESPE, Salt Lake City, Utah) was applied to all samples to bond the orthodontic brackets. Shear bond strength testing was carried out in a universal testing machine operating at 1.0 mm per minute.
Results
The results were statistically analyzed with 2-way analysis of variance (ANOVA) and Tukey tests ( P <0.05). Saliva contamination caused a significant decrease of enamel strength in the groups bonded with TB and SE compared with the SW and C groups. Enamel bond strengths of the C and SW groups were higher than those of the TB group, followed by the AD and SE groups. Enamel bond strength after S was higher than AD, followed by TB and SE.
Conclusions
The shear bond strengths of orthodontic brackets to enamel and the performance of the adhesive systems were influenced by contamination with saliva.
The evolution of adhesive systems is commonly based on their ability to promote long-term and reliable adhesion to dental substrates. In the conventional etch-and-rinse technique, enamel is demineralized with a mild acid (35%-37% phosphoric acid), allowing the mineral content to be rinsed away. Bonding is therefore accomplished by micromechanical interlocking between synthetic polymers and etched-enamel minerals.
Self-etching primers (SEPs) and adhesives are effective for orthodontic bracket bonding to enamel. These products reduce the number of steps in the bonding procedure, eliminating the necessity to rinse the etchant and dry the surface, thus simplifying the bonding technique. SEPs present a methacrylate phosphoric acid ester molecule composed of phosphoric acid and a methacrylate group. These groups are united in the acid ester molecule, and, as the primer is applied to enamel or dentin, the phosphate group dissolves and removes the calcium from the hydroxyapatite. Instead of being rinsed away, the calcium forms a complex with the phosphate group, and the complex is incorporated into the network during primer polymerization. Etching and monomer penetration of the exposed enamel rods is synchronized; therefore, the depth of etching is the same as that of primer penetration.
The results of combining conditioning and priming in a single stage of treatment are improved chair-side efficiency, cost savings for the clinician, and time savings for patients. In addition, the simple application of the SEPs might also decrease the possibility of saliva contamination between the conditioning steps; this is important for maintaining adhesive bond strength.
Some authors described the effect of saliva contamination on enamel using self-etching adhesive; according to these reports; contamination causes detrimental effects on enamel. Although saliva might allow clinically unacceptable bond strengths it seems to be a less problematic contamination than blood.
For the removal of saliva contaminants from the surface of enamel and dentin or the inner surfaces of restorations, phosphoric acid gel has been recommended (Kuraray Medical, Osaka, Japan), but chemical cleaning can also be achieved by using alkaline, acidic, or neutral solvent or emulsion. In clinical orthodontic bracket bonding situations, enamel contamination is common, since this procedure is performed with isolation by cotton pellets or cheek retraction. The contaminant is often removed with water followed by an air spray after enamel hybridization, but little is known about the efficacy of this procedure concerning enamel bonding strengths.
In view of the possible problems related to contamination of hybridized enamel, the objective of this study was to evaluate the effects of saliva contamination on shear bond strength of orthodontic brackets bonded with self-etching adhesive systems.
Material and methods
This study was done according to the ethical committee guidelines of the University of Taubaté approved by the National Health Council of Brazil (011/2004).
The experimental units consisted of 135 bovine incisor crowns embedded in self-curing polystyrene resin cylinders randomly divided into 9 groups of 15 samples each. The factors under study were adhesive system application and surface contamination. Adhesive systems were studied at 3 levels: Transbond Plus Self Etching Primer (TB) (3M Unitek, Monrovia, Calif), Adhese Single Bottle (AD) (Ivoclar, Vivadent, Schaan, Liechtenstein), and Self Etch Bond (SE) (Vigodent, Rio de Janeiro, Brazil). The second factor (surface contamination) was also studied at 3 levels: control group (C), no surface contamination; salivary contamination (S); and salivary contamination rinsed with deionized water (SW).
The response variable was orthodontic bracket bond strength measured by using a shear bond strength test.
One hundred thirty-five bovine incisors, free of defects, were stored in 0.1% thymol solution at 6°C for no longer than 2 weeks and used in this study. The teeth were cleaned of gross debris and stored in deionized water for 24 hours before the experiment. The roots were separated from their crowns by using a diamond disk (KG Sorensen, Barueri, São Paulo, Brazil) with a low-speed hand piece (Kavo do Brasil, Joinville, Santa Catarina, Brazil). The teeth were autoclaved (Cristófoli, Paraná, Paraná, Brazil) and embedded in autopolymerizing polystyrene resin (Cromex, São Paulo, São Paulo, Brazil) in a polyvinyl chloride ring mold (diamter, 2.0 cm; height, 2.0 cm) with the flat buccal enamel surface exposed. A jig was used to align the buccal surface of each tooth parallel to the cylinder’s base.
The exposed enamel was cleaned, and an area of 6 mm 2 was delimited. The samples were randomly divided into 3 groups, according to the adhesive treatment ( Table I ) applied according to the manufacturers’ recommendations. After the application of the adhesives, the samples were subdivided into 3 groups in which the enamel would or not be contaminated with artificial saliva (n = 15): C, S, and SW. The artificial saliva was Salivan (Apsen Pharmaceutics, São Paulo, Brazil).
| Adhesive system | Basic composition | Application mode |
|---|---|---|
| Transbond Plus Self Etching Primer (TB) |
Primer: methacrylate, phosphoric acid esters, initiator, stabilizer. Bond: water, fluoride complexes, stabilizer. |
Apply the primer to the enamel surface and rub for 3 s. Then give a gentle burst of dry air to thin the primer for 1-2 s. |
| Adhese Single Bottle (AD) | Primer: dimethacrylate, phosphoric acid acrylate, water, initiators, stabilizers. Bond: dimethacrylates, HEMA, SiO 2 , initiators, stabilizer. |
Apply primer to enamel for 15 s and remove excess, leaving a moist surface. Apply 2 consecutive coats of adhesive with a disposable saturated brush tip. Evaporate solvent with a mild air stream for 5 s and light cure for 10 s. |
| Self Etch Bond (SE) | Primer: HEMA copolymer, adhesive monomer (MEP), dimethacrylate, alcohol, water, photoinitiators and stabilizers. Bond: adhesive monomer (MEP), HEMA, BisGMA, alcohol, dimethacrylates, microfiller, photoinitiators, and stabilizers. |
Apply primer and massage the cavity for approximately 20-30 s. Do not wash and then gently apply a stream of air; do not desiccate the cavity. Apply the adhesive followed by a short stream of air and then light cure for 20 s. |
The buccal surface of each tooth was contaminated with a coat of approximately 1 mm of artificial saliva via air spray. The saliva contamination was carried out once, after adhesive application, for 60 seconds. Immediately after saliva contamination, the enamel surfaces of the S group were air dried for 15 seconds; in the SW group, the enamel was rinsed with deionized water for 15 seconds and air dried for 15 seconds. The C group received no contamination, and the SEP was applied according to the manufacturer’s instructions.
After bonding and treatment of the enamel surfaces, composite resin (Z100, 3M/ESPE, Salt Lake City, Utah) was applied to the base of the stainless steel orthodontic brackets (Morelli, Sorocaba, São Paulo, Brazil) and placed above the delimited areas on the tooth surfaces. All samples received a constant pressure of 150 g for standardization of the composite layer thickness by using a flat tip attached to a parallelometer (BioArt, São Carlos, São Paulo, Brazil). After removal of excess composite with a brush, the samples were light cured for 40 seconds, with a power density of 700 mW per square centimeter (Optilux 401, Demetron/Kerr, Danbury, Conn).
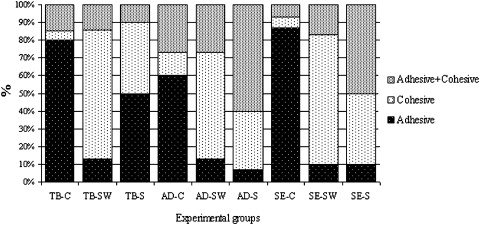
Shear bond strength testing was performed in a universal testing machine (Versat 2000, Pantec, Panambra, Brazil) operating at 1.0 mm per minute until bond failure. The debonded enamel sites were viewed under a stereoscopic loupe at 30-times magnification to assess the failure mode. Failure was classified as adhesive (up to 90% of the enamel surface exposed), cohesive (all adhesive remained on the tooth with a distinct impression of the bracket base), and mixed failure (also called adhesive and cohesive failure, indicating that up to 50% of the enamel surface was covered with adhesive or resin) ( Fig ).