Introduction
Despite rapid development in adhesive technology, contamination of bonding surfaces remains a major problem. The aims of this study were to evaluate the influence of contamination on bond strength and to investigate possible decontamination procedures.
Methods
Four bonding systems were evaluated for their shear bond strengths under 5 bonding situations: control (without contamination and decontamination); contamination with blood; contamination with saliva; decontamination with water and air, and repriming after blood contamination; and decontamination with water and air, and repriming after saliva contamination. The 25 specimens of each group consisted of composite blocks bonded to bovine teeth. Shear forces were measured with a testing machine after thermocycling.
Results
The 3 composite primers showed similar behavior. With the exception of Transbond SEP (3M Unitek, Monrovia, Calif) with saliva contamination, all contaminated samples showed greatly reduced shear forces. The control and decontaminated groups showed shear forces about 20 MPa. The resin-modified glass ionomer, however, did not reach clinically sufficient bond strengths in either setup.
Conclusions
Decontamination with water and air and repriming is sufficient after contamination with blood or saliva. Etching again is not necessary. The bond strength of Transbond SEP was not significantly altered by saliva contamination and can be recommended for conventional bonding procedures.
Since its introduction in 1955 by Buonocore, adhesive techniques have had tremendous success in diverse fields of dentistry. However, it is still difficult to maintain perfect bonding conditions for these techniques in the oral cavity. In particular, contamination with blood or saliva is frequent.
To overcome these problems, adhesives more tolerant to humidity have been developed in the last decade. One modification to achieve this is to add acetone or ethanol to the primer as a solvent. Both substances can displace water and should therefore be less sensitive to contamination. Clinically, the necessity for a dry environment still remains. Another class of substances that are known to be more hydrophilic than conventional composites are resin-modified glass ionomers. Ionic linkage between the hydroxyapatite and the carboxylate of the polycarbonic acid allows for application to unetched enamel. The additional application of a conventional etching agent, however, enhances bonding strength significantly.
Another possibility to minimize contamination is to reduce the clinical steps of the bonding procedure and thus eliminate sources of contamination, as has been done with self-etching primers. Acid monomers are used in self-etching primers. They dissolve the enamel surface and, by doing so, release calcium ions. This causes neutralization of the acid monomers, and thus the progression of etching is stopped, and equal penetration of etching and bonding is guaranteed. In addition, higher tolerance against contamination with water or saliva has been observed.
In addition to the maintenance of a dry environment, the development of humidity-tolerant adhesives, or the reduction of opportunities for contamination, a fourth approach can be considered: decontamination. Little is known about successful decontamination procedures after blood or saliva contamination of a previously etched or even an already bonded tooth surface. Because procedures involving renewed etching of tooth surfaces cause further enamel loss, we focused on the clinical possibilities of decontamination by water and air and repriming without renewed etching.
Many studies have evaluated the influence of contamination on shear bond strengths. The most critical times for contamination are immediately before and after the primer has been applied to the tooth. Contamination before priming would inevitably cause the formation of a smear layer. This layer consisting mainly of proteins covers the etched surface within seconds and thus inhibits the penetration of the porous surface by the priming agents. Contamination can also take place after priming. Fewer hydrophobic adhesive bonds will cause reduced adhesive strength. Both situations result in inferior adhesive strength and might lead to early detachment of the brackets or retainers.
Our aim was to evaluate which substance is least affected by contamination with saliva or blood after priming, and whether decontamination without renewed etching can reestablish acceptable bond strengths.
Material and methods
Four bonding systems were evaluated for their adhesive properties in different bonding situations ( Table I ). System 1 comprised etching with 35% phosphoric acid, Transbond XT primer and Transbond XT adhesive (3M Unitek, Monrovia, Calif). System 2 comprised the same etching procedure and adhesive, but the moisture-insensitive primer Transbond MIP (3M Unitek) was used. System 3 consisted of the self-etching primer Transbond Plus SEP (3M Unitek) combined with Transbond XT adhesive. In system 4, the resin-modified glass ionomer Fuji Ortho LC (GC America, Alsip, Ill) was used with 10% polyacrylic acid Ortho Conditioner (GC America).
| Contamination | Reconditioning | ||||
|---|---|---|---|---|---|
| Group | Etching | Priming | Control | No repriming | Bonding |
| Transbond XT and Transbond MIP (3M Unitek) |
35% phosphoric acid for 15 s, rinsing with water for 10 s, drying with air | Transbond XT/MIP primer for 10 s, blow of air | Contamination with blood or saliva for 10 s each | Repriming for 10 s with Transbond XT/MIP primer, blow of air | Bonding of composite blocks and light curing for 40 s |
| Contamination as above and decontamination with water for 10 s, drying with air | |||||
| Transbond Plus SEP (3M Unitek) |
No etching | Transbond SEP for 10 s, blow of air | Contamination with blood or saliva for 10 s each | Repriming for 10 s with Transbond Plus SEP, blow of air | Bonding of composite blocks and light curing for 40 s |
| Contamination as above and decontamination with water for 10 s, drying with air | |||||
| Fuji Ortho LC (GC America) |
GC Ortho conditioner for 20 s, rinsing with water for 30 s, drying with air | No priming | Contamination with blood or saliva for 10 s each | No repriming | Bonding of composite blocks and light curing for 40 s |
| Contamination as above and decontamination with water for 10 s, drying with air |
Each bonding system was divided into 5 groups according to the contamination and decontamination procedures: (1) ideal bonding condition, (2) contamination with blood, (3) contamination with saliva, (4) contamination with blood and decontamination with water and air followed by repriming without etching, and (5) contamination with saliva and decontamination with water and air followed by repriming without etching.
Each group consisted of 25 specimens, for a total of 500 shear force settings. Freshly extracted bovine deciduous incisors were used as the enamel substrate. The teeth were controlled for macroscopic deficiencies in the enamel structure. Only teeth with visually intact enamel surfaces were selected for testing. For hygienic reasons, the teeth were cleaned of soft tissue, and the pulp was extirpated. Thereafter, they were stored in physiologic sodium chloride for a week before shear bond strength testing.
The buccal surfaces of all teeth were pumiced. For systems 1 and 2 (Transbond XT and MIP primer), the teeth were etched for 15 seconds and rinsed for another 15 seconds. Whereas the specimens of system 1 were dried with air for 20 seconds, tooth surfaces of system 2 were kept moistened. Both primers were applied for 10 seconds and dispersed with an air flow for 5 seconds. In system 3 (Transbond SEP Plus), no etching was done. Transbond SEP was applied for 5 seconds and dispersed with an air flow for 2 seconds. In system 4, GC Ortho Conditioner was used for an etching period of 20 seconds, followed by thorough rinsing for 30 seconds. A slight humidity was preserved by gentle drying with air.
Whereas group 1 was the uncontaminated control group, groups 2 and 3 were contaminated with blood and saliva, respectively, for 10 seconds before application of the adhesive. Both blood and saliva were collected from the examiner (M.E.). Groups 4 and 5 were contaminated in the same manner as groups 2 and 3, but they were decontaminated with water for 10 seconds. Thereafter, these groups were reprimed according to the normal protocol. In system 4 (Fuji Ortho LC), the contamination and decontamination procedures were also performed before application of the adhesive. However, because there was no priming agent, the contamination was applied to the etched enamel.
Composite blocks with a bonding surface of 12.6 mm 2 and a height of 5 mm were used for shear bond testing. For this, highly precise cylinders were constructed from stainless steel by a computerized numerical control mill (Picomax 60-M/HSC, Fehlmann AG, Seon, Switzerland). A negative impression was taken by using low viscosity silicone (Finosil, Fino, Schweinfurt, Germany). The negative cylinders formed by the impression were filled with a flowable composite (Grandioflow, VOCO, Cuxhaven, Germany) and light cured (Bluephase, Ivoclar Vivadent, Lichetenstein).
After bonding the composite blocks to the teeth, the probes were subjected to 1000 temperature cycles between 5°C and 55°C within 50 hours (Circulator C-85, Techne, Stone, UK; Julabo UC and 5B, Julabo Labortechnik, Seelbach, Germany).
To obtain a bonding interface parallel to the shear force vector, a positioning gauge was used. The teeth were embedded in a polymethacrylate socket (Technovit, Heraeus Kulzer, Wehrheim, Germany) and tested for maximum shear force with a universal testing machine (model 4444, Instron, Wilmington, Del). In addition, the adhesive remnant index (ARI) scores were evaluated for all samples, by estimating the amount of bonding material remaining on the 2 surfaces under 3.5-fold magnification. A score of 0 was used for samples with no adhesive left on the enamel, 1 for less than 50% on the enamel, 2 for more than 50% on the enamel, and 3 when all adhesive remained on the tooth.
Statistical analysis
SAS software (version 9.1, SAS, Cary, NC) was used for statistical analysis. Means, medians, minimum and maximum values, and standard deviations were calculated. The nonparametric Kruskal-Wallis test was used to rank the results of the different groups. Significance was set at P <0.05 and calculated with the Mann-Whitney U test. Samples that could not be measured because of spontaneous detachment during thermocycling were evaluated as 0 bond strength.
Results
Mean shear force values were about 20 MPa for the control and the decontamination groups of Transbond XT, MIP, and SEP as well as the saliva contamination group of Transbond SEP. All other shear fractures occurred at about 5 MPa ( Fig , Table II ). The shear values showed a direct correlation to the ARI scores, achieving high ARI scores for groups with high bond strengths ( Table II ). This resulted in a correlation factor of r = 0.87 according to the Spearman nonparametric calculation. Fuji Ortho LC showed significantly lower values for the control group and both decontamination groups, whereas the 3 composite systems showed no significant differences between the control and decontamination groups or between the different materials ( Table III ). For the contamination groups, Transbond XT showed small but significantly higher bond strengths than its competitors, and Transbond SEP had highly significant advantages in bond strength with saliva contamination ( Table IV ).
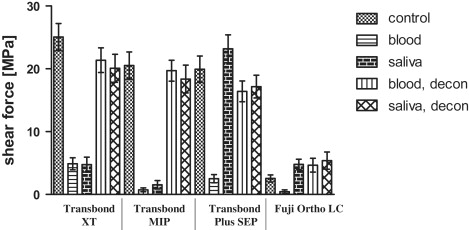
| System | Group | Mean (Mpa) | SD (Mpa) | ARI score | Enamel fracture (n) | Early detachment (%) |
|---|---|---|---|---|---|---|
| Transbond XT | 1 (control) | 25.06 | 10.81 | 2.2 | — | — |
| 2 (blood) | 4.88 | 4.82 | 0.44 | — | 12 | |
| 3 (saliva) | 4.75 | 5.88 | 0.56 | 1 | 24 | |
| 4 (blood, decon) | 21.37 | 9.81 | 2.12 | 2 | — | |
| 5 (saliva, decon) | 20.09 | 11.13 | 1.72 | 1 | — | |
| Transbond MIP | 1 (control) | 20.53 | 10.79 | 1.96 | 2 | — |
| 2 (blood) | 0.73 | 1.51 | 0.16 | — | 68 | |
| 3 (saliva) | 1.54 | 3.33 | 0.04 | — | 64 | |
| 4 (blood, decon) | 19.70 | 8.32 | 2.16 | 1 | — | |
| 5 (saliva, decon) | 18.37 | 11.04 | 1.68 | 1 | — | |
| Transbond Plus SEP | 1 (control) | 19.94 | 10.53 | 2.68 | 9 | — |
| 2 (blood) | 2.51 | 3.29 | 0.08 | — | 52 | |
| 3 (saliva) | 23.19 | 11.13 | 2.52 | 5 | — | |
| 4 (blood, decon) | 16.41 | 8.29 | 2.2 | 5 | — | |
| 5 (saliva, decon) | 17.17 | 9.09 | 2.04 | 2 | — | |
| Fuji Ortho LC | 1 (control) | 2.56 | 2.81 | 0.2 | — | 28 |
| 2 (blood) | 0.45 | 1.40 | 0 | — | 88 | |
| 3 (saliva) | 4.78 | 4.19 | 0.28 | — | 8 | |
| 4 (blood, decon) | 4.67 | 5.51 | 0.32 | — | 8 | |
| 5 (saliva, decon) | 5.38 | 6.89 | 0.16 | — | 28 |
| 1. Rank | P 1 | 2. Rank | P 2 | 3. Rank | P 3 | 4. Rank | P 4 | 5. Rank | |
|---|---|---|---|---|---|---|---|---|---|
| Transbond XT | Control | 0.21 | Blood decontaminated | 0.54 | Saliva decontaminated | <0.0001 | Blood | 0.63 | Saliva |
| Transbond MIP | Control | 0.71 | Blood decontaminated | 0.53 | Saliva decontaminated | <0.0001 | Saliva | 0.61 | Blood |
| Transbond Plus SEP | Saliva | 0.33 | Control | 0.43 | Saliva decontaminated | 0.90 | Blood decontaminated | <0.0001 | Blood |
| Fuji Ortho LC | Saliva | 0.62 | Blood decontaminated | 0.88 | Saliva decontaminated | 0.22 | Control | 0.0001 | Blood |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


