Introduction
An experimental analysis was made to quantify the adherence rates and the biofilm formation capacity of Streptococcus mutans ATCC25175 and Candida albicans SC5314 on orthodontic material surfaces in the presence of cigarette smoke condensate (CSC).
Methods
Metal brackets, bands, acrylic resin, and polyurethane elastic rings were coated with stimulated saliva and submitted to adhesion and biofilm formation tests with and without CSC in a dynamic system.
Results
The CSC increased the adhesion of S mutans ATCC25175 to the acquired pellicle ( P <0.05) for bands (4.08 times), acrylic resin (2.89 times), and brackets (3.37 times) and reduced it in polyurethane elastic (2.66 times; P <0.05). S mutans ATCC25175 biofilm biomass was increased by CSC only on brackets (1.60 times; P <0.05). In the presence of CSC, the adhesion of C albicans SC5314 increased ( P <0.05) on bands (1.81 times), brackets (9.61 times), elastics (29,133 times), and acrylic resin (177 times). Greater formation of C albicans SC5314 biofilm caused by CSC ( P <0.05) was observed on acrylic resin (2.13 times) and brackets (2.32 times).
Conclusions
The results indicated that cigarette tobacco smoke can interfere with the adhesion and biofilm formation of these microorganisms to various orthodontic materials.
According to reports of the Centers for Disease Control and Prevention in Atlanta, Ga, approximately 19.8% of adults and 20.0% of high school students in the United States were smokers in 2007. Certainly, these values do not differ significantly worldwide. These data are highly apprehensible, once the habitual consumption of tobacco is related with an uncountable number of serious morbidities such as lung emphysema, oral and pulmonary cancers, among others. Smoking is also a problem in dentistry. Orthodontists and other health professionals must ask their patients to stop smoking by pointing out the hazards of the addiction. For instance, chronic destructive periodontal disease in smokers is started and driven by smoking, and its progression might be amplified by unavoidable microbial colonization. Also, oral candidosis seems to be precipitated by impairment of the local immune system due to smoking. Although it is discussible, the role of smoking in the progression of caries must not be ignored when there is consistent evidence that smoking positively contributes to increased mutans populations.
On the other hand, dental caries is an undesirable effect of orthodontic therapy and can affect up to 50% of patients. Streptococcus mutans is the bacterium most commonly associated with the development of cavities and has a good capacity for adhesion and growth on orthodontic material surfaces.
Although there is no consensus, there are indications, albeit experimental, that Candida albicans might also be involved in carious processes. In spite of the polemic surrounding the subject, the placement of fixed orthodontic appliances alters the oral ecologic parameters with subsequent elevation of candidal counts.
Although some groups have investigated the adhesion of microorganisms and biofilm formation on bands, brackets, polyurethane elastics, and acrylic components, no study has evaluated the influence of cigarette smoke on these virulence factors.
Among the various microorganisms in biofilms formed on orthodontic materials, in this study, S mutans and C albicans were evaluated with regard to their adhesion and biofilm formation capacities in the presence of cigarette smoke condensate (CSC), because they are implicitly associated with several oral diseases.
The aim of this study was to experimentally analyze the adherence and biofilm formation capacity of S mutans ATCC25175 (American Type Culture Collection, Manassas, VA), and C albicans SC5314 (kindly provided by Prof. Lakshman P. Samaranayake, University of Hong Kong) on orthodontic material surfaces in the presence of CSC.
Material and methods
C albicans SC5314 was grown in 50 mL of yeast nitrogen base (YNB) (Difco Laboratories, Detroit, Mich) at 37 °C, 100 rpm, and normoxic conditions. After 24 hours, the cells were centrifuged and washed in sterile 145 mM sodium chloride. The cells were resuspended up to an OD 660nm of 0.8 (Ultrospec 1100-pro, Amersham Biosciences, Buckinghamshire, United Kingdom). In a similar manner, S mutans ATCC25175 was grown in 50 mL of brain heart infusion (BHI) (Difco Laboratories) at 37 °C, 100 rpm, and 10% (partial pressure of CO 2 ). After 24 hours, the cells were centrifuged, washed in sterile 145 mM sodium chloride, and resuspended up to an OD 660nm of 1.0.
The volunteers’ participation in the project was subject to signing an informed consent, in accordance with the guidelines of the Ethics Committee on Research in Human Beings of the Pontifical Catholic University of Paraná in Brazil. The aliquots of saliva (10 mL) were collected from 3 adults (age, 28.7 ± 1.7 y), 2 women and 1 man, with no systemic or perceptible oral disease, who were instructed not to eat or brush their teeth for at least 2 h before the collections. These collections were always made at 9:00 am to minimize the effects of circadian variability on saliva composition. Salivary stimulation was conducted mechanically by chewing 1-cm sections of sterile silicone tubes for 5 m, after discarding the residual saliva secreted in the first minute. The aliquots were mixed to form an acquired pellicle with a greater number of receptors. Immediately after collections, the samples were cooled on ice and purified by centrifugation (10,000 g , 4 °C, 20 m) to remove the impurities, such as bacteria and food particles, and were used immediately.
The volatile products in the CSC were collected by washing the smoke from the complete burn of 5 cigarettes containing 10 mg of tar, 0.8 mg of nicotine, and 10 mg of carbon monoxide per cigarette (Marlboro KS Box, Philip Morris, Santa Cruz do Sul, Brazil) in an in-house smoking machine containing 100 mL of distilled water ( Fig 1 ). The quantity of cigarettes and the volume of water were determined arbitrarily on the assumption that a smoker consumes 20 cigarettes in a 16-hour period of wakefulness, excluding 2.5 hours. The adhesion and biofilm formation tests were conducted with a constant concentration of CSC, because of the experimental difficulty of reliably mimicking variations of concentration in the residual saliva of smokers. This brand of cigarette was chosen because it is widely smoked worldwide.
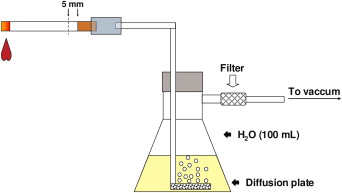
The water containing the CSC was filtered through a 0.44-μm pore membrane (Millipore, São Paulo, Brazil) immediately after the cigarettes were burned. The filtrate was fractioned into sterile flasks that received equal volumes of sterile YNB and BHI double concentrated. One part of the filtrate was not mixed with the broths and reserved for the adherence tests.
Various orthodontic materials were used to evaluate adherence: (1) stainless steel brackets, 0.022 × 0.028-in slot, prescription MBT for the maxillary right central incisor (TP Orthodontics, LaPorte, Ind); (2) strips of stainless steel GripTite Molar Bands (TP Orthodontics) cut into sections of 0.10 × 3.80 × 7.00 mm; (3) elastic ligatures of the modular Super Slick type (TP Orthodontics); and (4) strips of Jet acrylic resin measuring 0.10 × 4.00 × 7.00 mm (Classico, São Paulo, Brazil). The real dimensions of all materials were confirmed after images were captured with a measuring microscope (MM-40, Nikon Instruments, Melville, NY) under 50 times magnification. The images were processed by the metronics software QC-4000 for Windows (Automation and Metrology, Painesville, Ohio).
The materials were disinfected by multiple sonications (10 pulses of 30 sec and 50 kHz per washing) in closed vials containing sterile double-distilled water. The disinfections were confirmed after incubation of all specimens in BHI at 37 °C, normoxia, for 24 to 48 h. We observed no microbial growth after 5 sonication cycles. All specimens were washed with sterile double-distilled water and used in further steps.
The tests were conducted by performing 4 experimental treatments on the materials: (1A) S mutans ATCC25175 vs CSC, (2A) S mutans ATCC25175 vs distilled water, (3A) C albicans SC5314 vs CSC, and (4A) C albicans SC5314 vs distilled water. Each treatment was conducted with 4 specimens per material, in 6 independent repetitions, totaling 24 tests. Blanks consisted of materials covered solely by the acquired pellicle, which were submitted to the same experimental conditions, but without the microorganisms.
On flat-bottomed 96-well microtitration plates (TPP, Trasadingen, Switzerland) the various materials were immersed in a mixture of clarified saliva for 10 minutes. The materials were washed twice with sterile 145 mM NaCl to remove the excess saliva that was not used in the acquired pellicle formation.
In treatments 1A and 2A, the film-coated materials were left in contact with the suspension of S mutans ATCC25175 containing 10 8 colony-forming units (CFU) per milliliter for 2 hours at 37 °C, 10% pCO 2 , and 100 rpm. For treatments 3A and 4A, a suspension containing 10 7 CFU per milliliter of C albicans SC5314 was kept in contact with the materials, following the same incubation parameters as those used for S mutans ATCC25175.
The adhered cells were fixed with 99% methanol (Merck, Darmstandt, Germany) for 15 minutes. The materials were air dried and immersed in 0.5% crystal violet (CV) (Isofar, Rio de Janeiro, Brazil) for 20 minutes. The excess CV was removed by immersing the materials in distilled water twice. Finally, the impregnated CV was released by the addition of 250 μL of 33% acetic acid (Merck), and the OD 590nm was determined. The absorbance values of the blanks were subtracted from the values obtained in the treatment to eliminate spurious results from background interference.
The tests for interference in biofilm formation were conducted with the previously described orthodontic materials, submitted to 4 treatments: (1B) S mutans ATCC25175 vs BHI-CSC, (2B) S mutans ATCC25175 vs BHI, (3B) C albicans SC5314 vs YNB-CSC, and (4B) C albicans SC5314 vs YNB. Each treatment was conducted with 4 specimens per material, in 6 independent repetitions, totaling 24 tests. Blanks consisted of materials covered by the acquired pellicle, submitted to the same experimental conditions, but without the microbial loadings.
On flat-bottomed 96-well microtitration plates, the different materials covered by the acquired pellicle were left in contact with the suspension of S mutans ATCC25175 containing 10 8 CFU/mL for 2 hours, 37 °C, 10% pCO 2 , and 100 rpm (treatments 1B and 2B). For treatments 3B and 4B, a suspension containing 10 7 CFU/mL of C albicans SC5314 was kept in contact with the materials, with the same incubation parameters as those for S mutans . After the time required for microbial adhesion had elapsed, the specimens were carefully washed with sterile 145 mM NaCL and transferred to wells on another flat-bottomed 96-well microtitration plate. The wells were filled with BHI-CSC (1B), BHI (2B), YNB-CSC (3B), or YNB (4B) broth. The biofilms were formed in 10% pCO 2 for 72 hours, with careful changes of broth every 24 hours. The BHI and BHI-CSC broths did not receive any sucrose or other sugar, to reflect that a patient does not constantly have sugar in the saliva.
The biofilms formed after 72 hours were washed twice with sterile 145 mM NaCl. The materials were immersed in 99% methanol for 15 minutes. The materials were air dried and immersed in 0.5% CV for 20 minutes. The excess CV was removed by immersing the materials in distilled water twice. Finally, the impregnated CV was released by the addition of 250 μL of 33% acetic acid, and the OD 590nm was determined. The absorbance values of the blanks were subtracted from the values obtained in the treatment to eliminate spurious results derived from background interference.
Statistical analysis
The data obtained were tabulated in Excel spreadsheets (Microsoft, Redmond, Wash) and transferred to SPSS software (version 15.0, SPSS, Chicago, Ill). The results were tested regarding to the normality of distribution with the Kolmogorov-Smirnov test, and the equality of variances, with the Levene test. When the pairs with and without CSC showed normal distributions, the Student t test was used; in cases of nonnormal distribution, the Mann-Whitney U test was used. A P value of 0.05 was considered the threshold for statistically significant differences.
Results
To evaluate the level of interference of CSC in the adhesion and biofilm formation by the microorganisms studied, the comparisons between the experimental groups (with CSC) and the controls (without CSC) were performed in pairs, individually, by material. No simultaneous, multiple analyses were performed between different materials. A broader evaluation was intended and conceived by compiling the data obtained for the different materials.
The total area of the surfaces free for adhesion of bands (mean of 5 specimens) and acrylic resin stripes (mean of 5 specimens) was 27.6 ± 0.8 mm 2 , whereas the brackets (mean of 5 specimens) had a total exposed area of 21.4 ± 1.3 mm 2 , and the polyurethane elastic had 6.7 ± 0.6 mm 2 (mean of 5 specimens).
The adhesion of S mutans ATCC25175 to the acquired pellicle was significantly increased by the CSC ( P <0.05) for the band (4.08 times), acrylic resin (2.89 times), and bracket (3.37 times) ( Fig 2 ), but not for the polyurethane elastic, in which the CSC reduced the adhesion (2.66 times) ( P = 0.03411). When the data were compiled, independent of the nature or topography of the material, there was an increment in the mean rates of adhesion ( P <0.00001).
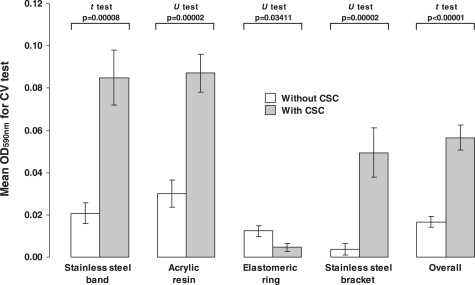
On the other hand, the continued exposure of S mutans ATCC25175 to the CSC led to greater biofilm growth on the bracket surfaces (1.60 times; P = 0.02727) ( Fig 3 ). The biofilms formed on bands, acrylic resin stripes, and polyurethane elastic were the same whether there was CSC or not ( P >0.05).