Introduction
Studies show that ceramic brackets are chemically inert in the oral cavity, whereas polycarbonate and polyoxymethylene brackets can degrade, releasing bisphenol-A and formaldehyde, respectively. In addition to the traditional cytotoxicity tests, the study of nitric oxide cellular production stimulated by a specific material has been shown to be a reliable tool for evaluating its cytotoxic potential.
Methods
We aimed to assess cellular viability by MTT (Sigma, St. Louis, Mo): 3,(4,5-dimethylthiazol-2-yl)-2,5diphenyl tetrazolium bromide assay in a murine macrophage cell line J774 with esthetic brackets and quantify nitric oxide production by these macrophages. Cell cultures were evaluated at 3 times: 24, 48, and 72 hours.
Results
Cellular viability in all groups was higher at 72 hours compared with 24 hours. This increase was significant in the control and ceramic brackets groups. Final means in the bracket groups showed no significant differences compared with the control group. Nitric oxide production was significantly greater in all groups at final time. There was no significant difference between the final means of the bracket groups and the control group, although polyoxymethylene brackets showed significantly greater means at 24 and 48 hours.
Conclusions
Final means in the bracket groups showed no significant differences compared with the control group.
In orthodontic therapy, dental materials classified as temporary are used for a medium or long period of time. Ceramic and plastic brackets were developed to meet the increasing demand for more esthetic appliances.
During treatment, a bracket-oral mucosa interface is created in the oral cavity; it can predispose both to alteration. This interface determines an active biologic response to the material, the biocompatibility is related to the material’s capacity to resist degradation. This behavior is intimately related to the composition, pretreatment, and handling of the materials.
All currently available ceramic brackets are made from alumina, which exists in nature in monocrystalline and polycrystalline forms. The primary advantage of single-crystal manufacturing is the elimination of possible stress-inducing impurities or imperfections. Ceramic brackets are chemically inert to oral fluids and show little reactivity with the oral environment. Nishio et al, however, stated that ceramic brackets are bulkier and have more contact area between the bracket and the oral mucosa. In addition, manufacturing procedures, finishing, and polishing of these brackets are difficult; this might explain their granular and pitted surfaces, which, in contact with the orthodontic wires, can jeopardize esthetics and biocompatibility.
Although polycarbonate (PC) brackets theoretically derive from inoffensive hyaluronic acid, they are considered much more potentially harmful than their composition suggests. The reason is that they are also derivatives of bisphenol A (BPA), which is used in the manufacture of PCs, resins, and fungicides. Recognized for over 70 years as an estrogenic, its derivative use in health-related fields is controversial. Allegations have been made that it triggers premature puberty in young girls and affects male fertility. Watanabe et al evaluated the degradation of PC brackets and the formation and release of BPA in vivo and in vitro. Some PC brackets were kept in the oral cavity for 18 to 40 months, and others were maintained in water at 37°C for 34 months. PC alterations were examined by means of high performance liquid chromatography. More BPA appeared to be released in saliva than was expected from the in-vitro data.
A third type of esthetic bracket is made of plastic: polyoxymethylene (POM), also called acetal resin, polyacetal, or polyformaldehyde. Kusy and Whitley concluded from their study of POM brackets that, despite their mechanical advantages, they have a propensity to depolymerize into formaldehyde. This could occur thermally (excessive heat) or chemically (alkalis, oxygen, or enzymes), or by radiolysis (during gamma radiation from x-ray scans or electron beam bombardment). Since degradation of POM brackets results in the release of formaldehyde, the authors concluded that the use of POM for orthodontic brackets, crowns for children, and other prosthetic appliances should be contraindicated because even radiography promotes its degradation. Zilberman and Eliades stated that no studies in the literature have reported the cytotoxicity or allergenic reactions from the release of formaldehyde in the oral cavity. According to those authors, the conclusion of Kusy and Whitley should be based on more reliable studies than those described here and by clinical studies of the potential toxicity of POM products compared with the alternative—usually metals containing nickel.
The International Organization for Standardization and the Council on Dental Materials, Instruments and Equipment of the American Dental Association have recommended batteries of in-vitro and in-vivo tests to study the biocompatibility of materials, and, according to the standards, the major tests for the initial evaluation of materials are cellular viability tests. Among these tests, the 3,(4,5-dimethylthiazol-2-yl)-2,5diphenyl tetrazolium bromide (MTT; Sigma, St. Louis, Mo) assay is an important test to determine the cytotoxicity of materials of different origins on cell cultures.
Cytotoxicity might also be related to molecules released by a certain stimulus and cause tissue alteration. Studies have reported a potentially cytotoxic molecule, nitric oxide (NO), in the tissues of the oral cavity and its involvement in inflammatory diseases. NO is a regulatory molecule, primarily produced by activated macrophages and is of paramount importance in the processes of immune response, inflammation, osseous metabolism, and apoptosis. This gas appears to be beneficial as well as detrimental. Beneficial effects can include antimicrobial activity and immune modulation. Its detrimental effects are cytotoxic actions toward adjacent host tissues, including alveolar bone.
The aims of this study were to assess in vitro the cellular viability with the MTT assay in a murine macrophage cell line J774 with ceramic, PC, and POM brackets, and also their effects in the production of NO by this macrophage line.
Material and methods
The study sample consisted of 6 ceramic brackets, 6 PC brackets, and 6 POM brackets for each time interval. They were used as bought commercially and were sterilized with ethylene oxide.
We used the murine cell line J774 A.1 (ATCC no. TIB-67, American Type Culture Collection, Manassas, Va), which was placed in a plastic bottle with a supplemented culture medium (5% bovine fetal serum, 50 IU per milliliter of penicillin, 1% nonessential amino acid, and 2% L-glutamine (Gibco, Grand Island, NY), and incubated at 37°C in 5% carbon dioxide. After transfer to an adequate container, the cells were washed with centrifugation at 1200 rpm, for 10 minutes at 4°C.
For determination of viability and cell count, Trypan blue stain was used in a 1:1 proportion (stain:culture medium) in the Neubauer chamber.
Culture plates (96 wells) were seeded with 2 × 10 4 J774 cells per well, in a volume of 100 μL, resuspended in culture medium supplemented with Roswell Park Memorial Institute Supplemented Medium 1640 (RPMI). Brackets were placed on the cells and kept in the culture for 3 time intervals (24, 48, and 72 hours) in 5% carbon dioxide at 37°C. After each incubation period, supernatant was collected for posterior NO quantification, and the cells were evaluated for cytotoxicity after bracket removal. The control group consisted of J774 murine macrophages, which were seeded in 96 well plates without any materials.
After supernatant removal, the brackets were removed with a sterile clamp. To the cells that remained in the wells of the plate, 90 μL of RPMI-supplemented medium and 10 μL of MTT solution (50 mg/mL) were added. Cells were incubated for 4 hours in a carbon dioxide oven at 37°C. After the incubation period, MTT reaction was blocked with 100 μL per well of alcohol-acid solution incubated for 10 minutes at room temperature, and the reading was done at 540 nm with a microplate reader (Spectramax 190-Molecular Device, Sunnyvale, Calif).
To evaluate NO production, 100 μL of supernatant from each well of the culture plate was transferred to 96 new well plates. The same amount of Griess reagent (1% sulfanilamide, 0.1% naphthylenediamine dihydrochloride, and 2.5% phosphoric acid) was added to the supernatant. Nitrite concentrations in the supernatants were obtained by linear regression analysis of the standard curve by using serial double dilutions of sodium nitrite from 200 μmol/L to the eleventh dilution. Absorbance was determined at 540 nm by using the microplate reader.
Statistical analysis
The statistical analysis was performed with Kruskal-Wallis and Mann-Whitney tests, by comparing the values obtained for each group of brackets with the control group. Results at 24 to 48 hours, 48 to 72 hours, and 24 to 72 hours were also compared for each material.
Results
Table I shows the P values of the Kruskal-Wallis test for cellular viability according to time ( P <0.05 for 48 hours) and material ( P <0.05 for the control and ceramic bracket groups).
| Time | P value | Material | P value |
|---|---|---|---|
| Control group | 0.001 | ||
| 24 hours | NS | Ceramic brackets | 0.001 |
| 48 hours | 0.001 | Polycarbonate brackets | NS |
| 72 hours | NS | Polyoxymethylene brackets | NS |
Table II gives the means and standard deviations for the analysis of cellular viability for each material at 24, 48, and 72 hours, and the P values of the Mann-Whitney test for the comparison of each material with the control group and for the comparison of cellular viability between 24 and 48 hours, 24 and 72 hours, and 48 and 72 hours for each group.
| Groups | Time | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|
| Control | Mean | 0.271 | 0.160 | 0.488 | |
| SD | 0.043 | 0.012 | 0.131 | ||
| P value between times | 24 h–48 h | 0.002 | |||
| 24 h–72 h | 0.009 | ||||
| 48 h–72 h | 0.002 | ||||
| Ceramic brackets | Mean | 0.242 | 0.188 | 0.387 | |
| SD | 0.023 | 0.013 | 0.166 | ||
| P value in relation to control | NS | 0.015 | NS | ||
| P value between times | 24 h–48 h | 0.002 | |||
| 24 h–72 h | 0.004 | ||||
| 48 h–72 h | 0.002 | ||||
| Polycarbonate brackets | Mean | 0.253 | 0.280 | 0.313 | |
| SD | 0.058 | 0.047 | 0.082 | ||
| P value relation to control | NS | 0.002 | NS | ||
| P value between times | 24 h–48 h | NS | |||
| 24 h–72 h | NS | ||||
| 48 h–72 h | NS | ||||
| Polyoxymethylene brackets | Mean | 0.206 | 0.207 | 0.272 | |
| SD | 0.028 | 0.081 | 0.304 | ||
| P value in relation to control | 0.041 | 0.002 | NS | ||
| P value between times | 24 h–48 h | NS | |||
| 24 h–72 h | NS | ||||
| 48 h–72 h | NS | ||||
The control group, the ceramic and PC brackets showed P <0.05 at 48 hours. POM brackets showed P <0.05 at 24 and 48 hours. Figure 1 also shows the comparisons between the groups of materials and the control.
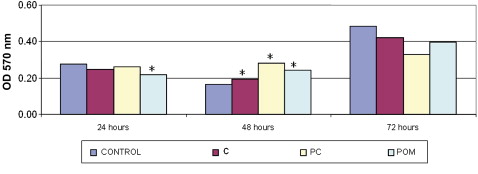
In the analysis of differences between the times for each material, both the control and the ceramic bracket groups showed P <0.05 at the 3 times evaluated. The PC and POM brackets had no statistically significant differences at any time.
Table III gives the P values of the Kruskal-Wallis test for NO production according to time ( P <0.05 for 24 hours) and material ( P <0.05 for all groups studied).
| Time | P value | Material | P value |
|---|---|---|---|
| Control group | 0.001 | ||
| 24 hours | 0.045 | Ceramic brackets | 0.002 |
| 48 hours | NS | Polycarbonate brackets | 0.003 |
| 72 hours | NS | Polyoxymethylene brackets | 0.001 |
Table IV shows the means and standard deviations for the analysis of NO production for each material at 24, 48, and 72 hours, and the P values of the Mann-Whitney test for the comparison of each material with the control group and the comparison of NO production between 24 and 48 hours, 24 and 72 hours, and 48 and 72 hours for each group. There was no significant difference for the ceramic and PC bracket groups when compared with the control group. The POM brackets showed P <0.05 at 24 and 48 hours. Figure 2 also shows the comparison between the groups of materials and the control group.



