Introduction
Our objective was to evaluate the effect of overloading on the palatal movement of the maxillary molar.
Methods
The maxillary first molars of male C57Bl/6 mice were moved palatally with loads of 10 or 30 g for 14 days, and the amount of tooth movement was longitudinally measured on microcomputed tomography images. Bone remodeling around the molar root with the 30-g load was evaluated at days 3, 5, 7, and 14 after the start of tooth movement using histomorphometry and immunodetection of bone-restricted interferon inducible transmembrane-like protein, a novel marker of active bone formation.
Results
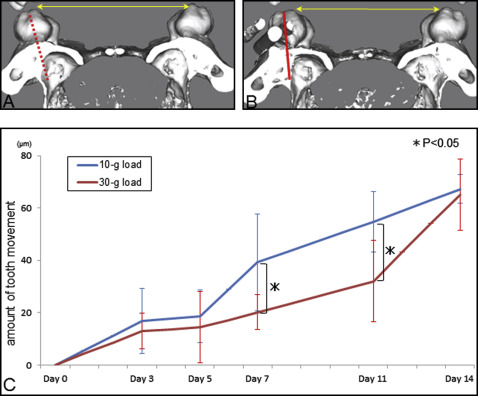
In the 10-g load group, the amount of tooth movement increased dramatically between days 5 and 7 and increased gradually thereafter. Tooth movement at days 5 and 7 was significantly lower in the 30-g-load group than in the 10-g load group; however, the total tooth movement at 14 days was similar in the 2 groups. An orthodontic load of 30 g stimulated bone formation on the sinus wall, but bone resorption on the periodontal ligament side was delayed because of hyalinization, which means that strong force application did not accelerate tooth movement. Moreover, some root resorption was induced by the excessive force.
Conclusions
Root penetration into the sinus and bone height reduction do not occur because new bone formation on the maxillary sinus is induced before bone resorption on the periodontal side, even though an excessive orthodontic force is applied. However, an excessive force can induce root resorption.
Highlights
- •
Mechanical stress applied to molars induced osteogenesis on the sinus wall.
- •
Palatal tooth movement did not lead to root penetration into the sinus.
- •
Palatal tooth movement did not lead to bone height reduction.
- •
Excessive loading will not accelerate tooth movement to the sinus.
- •
Excessive loading will induce more root resorption.
Contemporary orthodontics can provide 3-dimensional (3D) tooth movement to treat various malocclusions. However, it is generally believed that orthodontic tooth movement can be achieved only when healthy periodontal tissues and sufficient bony support are present in the direction in which the teeth will be moved. Therefore, tooth displacement in bone-deficient areas such as the maxillary sinus and the atrophic alveolar ridge is considered a major limitation in clinical orthodontics.
As for tooth movement through the maxillary sinus, Wehrbein et al pointed out the possibility of reducing the alveolar bone height or the root length in their dog experiment and human biopsy study. They suggested that the differentiation of osteoblasts required for compensatory subperiosteal bone apposition may be impaired by the structure and the specific metabolic condition of the mucosa in the maxillary sinus. In contrast to this common assumption, several reports have demonstrated that a tooth can be moved through the maxillary sinus while maintaining pulp vitality and bone support and exhibiting normal width of the periodontal ligament (PDL).
These conflicting views should be seriously discussed because of the emergence of implant-anchored orthodontics. In this decade, the development of temporary skeletal anchorage devices dramatically changed treatment strategies in clinical orthodontics. Temporary anchorage devices provide absolute anchorage and enable tooth movement considered to be difficult with conventional mechanics. Especially, absolute molar intrusion and group distalization of the dentition are considered to be leading-edge innovations. These tooth movements might increase the chance for root proximity or penetration into the maxillary sinus.
Recently, we evaluated aspects of tissue remodeling with palatal tooth movement using an experimental tooth movement (ETM) model with mice and found that the maxillary molar could be moved into the sinus safely because of osteogenesis in the sinus induced before bone resorption on the PDL side. However, the amount of force application on the teeth is suggested to be closely related to the safety of tooth movement. The optimal force is affected by many factors, such as root morphology, type of tooth movement, periodontal membrane area, and alveolar bone in the direction of tooth movement. Clinicians must be aware of the possibility of applying excessive orthodontic force on translatory tooth movement into the sinus unintentionally and know what happens after the overloading histologically. It is generally accepted by orthodontists that heavy forces produce significantly more root resorption than do light forces or controls. Therefore, overloading might prevent tooth movement and cause destruction of the periodontal tissues. However, few reports have evaluated in detail the effects of overloading in palatal tooth movement that moves roots into the sinus.
In this study, we have exploited a well-established ETM model to evaluate the effect of overloading on the palatal movement of the maxillary molars. Tooth movement was evaluated by microcomputed tomography (micro-CT) and histology of periodontal tissues on the compression area in the maxillary sinus using immunolocalization with bone-restricted interferon inducible transmembrane-like protein (Bril), an osteoblast-specific membrane protein associated with active bone formation.
Material and methods
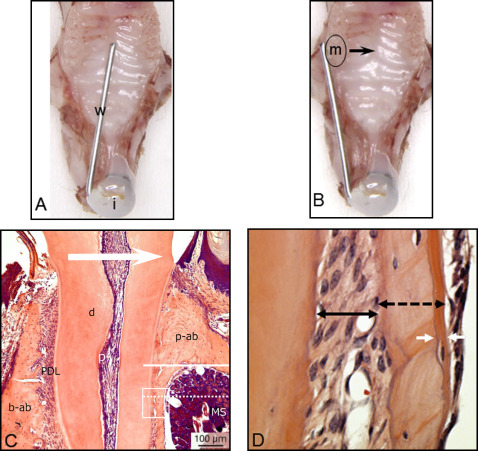
Thirty male C57Bl/6 mice (CLEA Japan, Tokyo, Japan) weighing 25 ± 5 g were anesthetized with an intraperitoneal injection of 1 mg per gram of pentobarbital (Somnopentyl; Kyoritsu Seiyaku, Tokyo, Japan). Nickel-titanium alloy wires (diameter, 0.012 and 0.016 in) were fixed to the maxillary incisors with composite resin for orthodontic bonding (Beauty Orthobond; Shofu, Kyoto, Japan) to achieve loads of 10 and 30 g, respectively, as shown in Figure 1 , and to move the maxillary left first molar toward the palatal side. Ten animals served to measure tooth movement in micro-CT analysis, and the other 20 were for the histologic analysis. All appliances were retained until the mice were killed. The untreated contralateral tooth served as the control. The mice were kept at a constant ambient temperature (22°C-24°C) with a day-night rhythm and fed a powdered diet ad libitum.
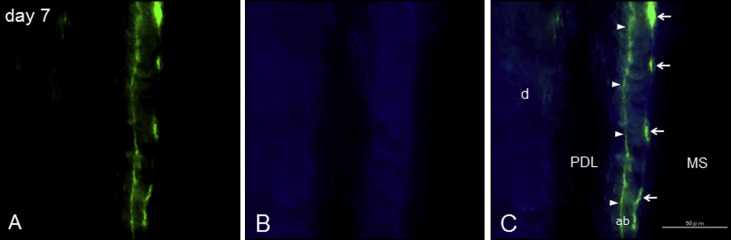
After 24 hours of force application and 1 day before they were killed, the mice for histologic analysis were intraperitoneally injected with 0.1 mL of calcein (2.5 g/L) (Wako Pure Chemical Industries, Tokyo, Japan) solution in 2% sodium hydrogen carbonate for fluorochrome labels. The experimental protocol described below was approved by the ethical committee of Tokushima University (permit number 11113).
At the start of tooth movement (day 0) and 3, 5, 7, 11, and 14 days after force application (10 or 30 g), micro-CT (LCT-200; Hitachi Aloka Medical, Tokyo, Japan) images of the maxillae were taken with inhalation anesthesia with isoflurane (Forane; Abbott, Tokyo, Japan) and 100% oxygen. The tube voltage was set at 80 kV, and the current was constant at 0.5 mA. The mice were scanned in a 48-mm-wide specimen holder with a resolution of 24 × 24 μm 2 pixel size. The data were saved in DICOM format, and the 3D reconstruction models were generated using 3D visualization and simulation software (ZedView, version 6.0; LEXI, Tokyo, Japan). On the 3D models, the distance between the mesiolingual cusps of the maxillary first molars was measured ( Fig 2 , A and B ). The intermolar width before tooth movement was used as the baseline. Each group for this analysis consisted of 5 mice.
At 3, 5, 7, and 14 days after the start of tooth movement, the animals were anesthetized with 1 mg per gram of pentobarbital and killed by perfusion through the left ventricle with phosphate buffered saline solution (PBS; Mitsubishi Chemical Medience, Tokyo, Japan) for 30 seconds, followed by a fixative solution consisting of 4% paraformaldehyde (Wako Pure Chemical Industries) and 0.1% glutaraldehyde (Katayama Chemical, Osaka, Japan) in 0.08 mol per liter of sodium cacodylate (Wako Pure Chemical Industries) buffer containing 0.05% calcium chloride (Junsei Chemical, Tokyo, Japan), pH 7.2, for 20 minutes. The maxillae were dissected, and the specimens were immersed in the same fixative solution overnight at 4°C and embedded into methylmethacrylate resin (Technovit 9100; Kulzer, Wehrheim, Germany). The resin blocks were sectioned at 5-μm thickness. The other specimens were decalcified with a solution consisting 5.4% formic acid (Kanto Chemical, Tokyo, Japan) and containing 0.4 mol per liter of trisodium citrate dehydrate (Wako Pure Chemical Industries) for 7 days at 4°C. The decalcified samples were washed for 24 hours in 0.1 mol per liter of sodium cacodylate buffer, pH 7.2, processed for paraffin embedding, and sectioned at 5-μm thickness. For the morphologic observations, the sections were stained with hematoxylin and eosin.
To evaluate the bone activity, we immunolocalized an osteoblast-specific membrane protein, Bril. The sections were deparaffinized with xylene, rehydrated through a decreasing ethanol series, and washed in distilled water. To prevent nonspecific sticking, the sections were blocked with 0.01 mol per liter of PBS, pH 7.2, containing 5% skim milk for 30 minutes at room temperature. After blocking, the sections were incubated with an affinity-purified rabbit primary antibody raised against Bril (1:5000, 3 hours, room temperature). The sections were washed with PBS containing 0.05% (v/v) Tween 20 (Bio-Rad Laboratories, Hercules, Calif), pH 7.4 (0.01 mol/L of PBS-Tween 20), followed by treatment with the Dako Envision TM+ System, HRP-labeled polymer antirabbit kit (Dako, Glostrup, Denmark), as recommended by the manufacturer. Visualization was performed with 3,3′-diaminobenzidine, and the sections were counterstained with 0.5% methyl green (Dako).
The histologic examination focused on the bone and the PDL surrounding the mesial root of the maxillary first molar at the palatal side. Each group for histomorphometry consisted of 5 mice. The area located about 100 μm from the palate level was evaluated in each animal. Newly formed bone was defined as the distance between 2 calcein lines under a fluorescence microscope (BZ-9000; KEYENCE, Osaka, Japan) ( Fig 3 ). The width of the PDL, total thickness of the bone, and newly formed bone were measured with image-editing software (ImageJ; National Institutes of Health, Bethesda, Md) ( Fig 1 , C ).
Statistical analysis
Homoscedasticity of variance was confirmed by F test, and the unpaired t test was used to compare the amount of tooth movement between the 10-g and 30-g-load groups. The significance of differences in the histomorphometric measurements was analyzed by 1-way analysis of variance and the Tukey-Kramer test. Probability levels of P <0.05 were considered statistically significant.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


