Biological solutions for the repair and regeneration of the dental tissues offer significant potential for improved clinical treatment outcomes. Translation of dental tissue-engineering approaches to the clinic will make considerable contributions to these outcomes in the future, but exploiting the natural regenerative potential of dentin-pulp to enhance wound-healing responses offers solutions for maintaining pulp vitality now. Strategies to harness the natural regenerative potential of the pulp must be based on a sound biological understanding of the cellular and molecular events taking place, and require careful consideration of the interplay of infection, inflammation, and regeneration.
- •
Biological solutions to the repair and regeneration of the dental tissues offer significant potential for improved clinical treatment outcomes.
- •
Translation of dental tissue-engineering approaches to the clinic will make considerable contributions to these outcomes in the future, but exploiting the natural regenerative potential of dentin-pulp to enhance wound-healing responses offers solutions for maintaining pulp vitality now.
- •
Strategies to harness the natural regenerative potential of the pulp must be based on a sound biological understanding of the cellular and molecular events taking place, and require careful consideration of the interplay of infection, inflammation, and regeneration.
Regenerative medicine offers many advantages for the treatment of disease with its aim of “replacing or regenerating human cells, tissues or organs to restore or establish normal function,” and within dentistry there is exciting future potential for engineering whole teeth. Nevertheless, several challenges still exist before such tooth-replacement therapies can be clinically implemented in dental practice. Paramount among these challenges is programmed development of appropriate crown and cuspal morphology for occlusal function and engineering of the tooth/periodontium interface to allow normal oral function. Use of scaffolds of defined morphology may assist with overcoming some of the morphologic challenges. Recently the authors generated a mineralized tissue construct, retaining the morphology of the human tooth used as a mold for production of an alginate scaffold within which dental pulp cells were seeded ( Fig. 1 ). Such approaches may contribute to realization of the engineering of whole teeth, although this remains a future goal for dentistry.
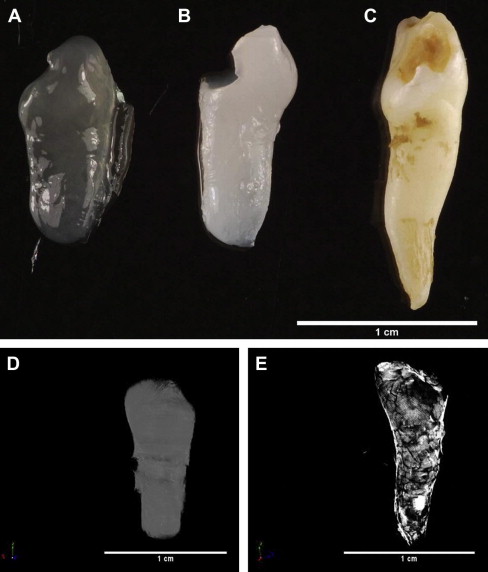
Considerable recent interest in the concept of regenerative endodontics has led to reports of engineering of individual dental component tissues, which provides a more achievable goal in the shorter term. Seeding of pulp cells isolated from exfoliated primary teeth in a simple polylactic acid scaffold within a dentin slice before subcutaneous implantation in vivo has allowed engineering of tissue with the same appearance and structure as normal pulp. A scaffold of collagen I gel containing isolated dental pulp cells and angiogenic growth factors within a pulpless tooth chamber showed evidence of revascularization and tissue generation after in vivo implantation. Although these tissue-engineering approaches may ultimately provide shorter-term solutions to the management of gross dental disease than whole tooth replacement, there are already viable regenerative endodontic techniques available to the clinician. Dentistry has long been a pioneer in regenerative medicine with the use of direct pulp-capping techniques for vital pulp therapy. Agents such as calcium hydroxide have been used for more than 90 years to stimulate natural pulp wound healing, although lack of understanding of the mechanistic basis of such treatments has perhaps led to their somewhat empiric application. Newer pulp-capping materials such as mineral trioxide aggregate (MTA) have demonstrated considerable clinical merit and, with appropriate strategic planning with regard to their clinical application, these agents can offer regenerative therapies to the practitioner now. However, opportunities exist to improve and optimize the use of such agents, and this will be important in bringing a more regenerative philosophy to the endodontics arena.
Natural wound healing essentially represents one end of the spectrum of tissue regeneration, and promotion of vital pulp therapy relies on principles applicable to healing of any of the body’s tissues. Promoting wound cleansing to enable the body’s natural defense responses of inflammation and immune reactions to take place in a controlled manner and not to become self perpetuating, however, can be particularly challenging in the tooth where the tissues are exposed to significant bacterial infection. Furthermore, the low-compliance nature of the dentin-pulp complex encased by rigid mineralized tissues followed by the introduction of dental restorative materials, sometimes possessing cytotoxic properties, may inhibit natural tissue-repair responses. It is perhaps not surprising that the prognosis for long-term survival of dental restorations corresponds poorly with desired health care outcomes, and mean survival rates of the order of 50% to 60% after 5 to 10 years have been reported. Together, such findings highlight the opportunities for development of new approaches to management of dental disease, whose success will be significantly enhanced by using new treatment modalities based on the cellular and molecular events associated with the healing responses of the dentin-pulp complex following injury.
These healing responses will be very dependent on the tissue environment resulting from the injury to the tooth and will often guide treatment-management decisions. For instance, it has been recommended that vital pulp therapy should only be attempted in primary and young permanent teeth where there is a good prognosis for pulp regeneration, and not in teeth with irreversible pulpitis or a necrotic pulp. Of course, this belies a fundamental issue of accurately diagnosing the status of the pulp with a limited armory of clinical tools, which do not necessarily correlate well with more biological measures. Nevertheless, it is well recognized that caries can present as a broad spectrum of disease activity, with consequent influence on the pulp and dental hard tissues and their regenerative potential. A regenerative response in the form of tertiary dentin secretion can be observed during slowly progressing caries but not rapidly progressing caries, highlighting the influence of microbial challenges to regeneration. However, it is not only the direct effects of the bacteria that are responsible for these responses, but also their indirect effects on various other events contributing to the overall regenerative picture. Of particular importance is the role of bacteria in triggering inflammatory and immune responses in the pulp, which in turn will affect pulpal regeneration. It is now apparent that the dentin matrix contains a heterogeneous cocktail of growth factors and cytokines, which may be released during bacterial acid demineralization of the matrix during caries, and these molecules will significantly contribute to the regenerative responses taking place. Surgical intervention to remove and restore carious hard tissue from the tooth may further contribute to tissue injury and cellular responses, in view of the intimate relationship between odontoblasts and their extracellular matrix. The extensive network of odontoblast processes and their lateral extensions in dentin permeates the whole tissue, and any surgical intervention to dentin is likely to provoke a cellular response. This premise is corroborated by data on odontoblast viability beneath cavities prepared to different depths through dentin, which indicate that survival is compromised in deeper cavities. Thus, there is a complex interplay between factors determining the environment within which regeneration may occur ( Fig. 2 ).
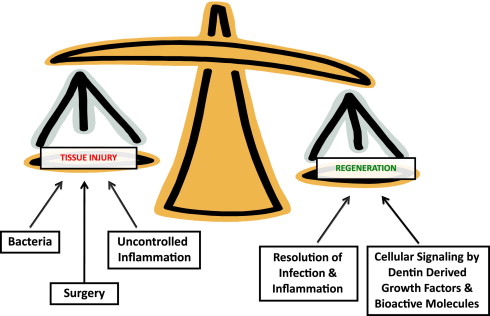
For regeneration to occur in the dentin-pulp complex, there is both a requirement for cells to secrete new tissue and for appropriate molecular and cellular signaling to occur, to enable cells to secrete the newly regenerated tissue. One can therefore envisage regeneration being determined by the following:
- •
A permissive environment in which regeneration can occur
- •
Cells capable of secreting the extracellular matrices of the dentin-pulp complex
- •
Secretion of molecular signals and cell-cell interactions to occur for upregulation of cellular synthesis and secretion of new dentin-pulp tissue.
Creating a conducive environment for regeneration
Microbial Challenges
The diverse microflora and variable intensity of infection in dentin caries provide significant clinical challenges for both assessment of the level of disease activity and control of the infection. The clinical picture for dentin caries largely provides evidence of previous rather than current disease activity, and without longitudinal review it can be difficult to assess current disease status. Such information can easily lead to greater clinical intervention than is either required or desirable. The traditional approach of removing as much infected, and at-risk, tissue as possible (Black’s “extension for prevention” philosophy) has possibly led to increased tissue injury with consequent effects on preservation of pulp vitality in the battle to control infection. Modern approaches of minimally invasive dentistry have helped to moderate the effects of surgical intervention but to increase the risk for restoration failure caused by secondary (recurrent) caries, whereby incomplete removal of residual caries during restoration can be a contributory factor. It is clear, however, that good clinical outcomes can be achieved using minimally invasive techniques despite the increased chance of incomplete removal of infected tissue. This outcome may well reflect the antibacterial effects of the agents and materials used in restorative procedures combined with the host’s defense mechanisms. Several chemicals, including sodium hypochlorite, various acids, ethylenediaminetetraacetic acid (EDTA), chlorhexidine, and so forth, are commonly used during cavity preparation and as irrigants in endodontics, and will exert antibacterial effects to varying degrees. Within adhesive dentistry, antibacterial agents are already being incorporated within dentin primers. Some materials, such as calcium hydroxide and Portland cement–like materials (eg, MTA), generate a strongly basic pH during setting, which will likely contribute to their antibacterial effects. Constituents of the dentin matrix also demonstrate antibacterial effects and, while these effects appear to derive from a heterogeneous mixture of molecules, including neuropeptides and cytokines such as adrenomedullin, there is still much to learn as regards the range of such molecules involved. These antibacterial activities may not necessarily be the prime functions for some of these molecules, but their release (and also their breakdown products) during carious demineralization of dentin will nevertheless expose such roles. It is thus clear that there is a need for a more focused therapeutic approach to microbial control in the carious tooth to promote regenerative activity.
Inflammatory and Immune Challenges
A complex range of inflammatory and immune responses can occur in the dental pulp in response to carious injury and subsequent clinical treatment involving many molecular mediators. While recognized as a terminally differentiated matrix-secreting cell, the odontoblast can participate in expression of various inflammatory mediators, and together with the strategic position of this cell in relation to carious bacterial invasion it should be considered as a part of the tooth’s cellular defense system. The complexity of the repertoire of the odontoblast’s responses, however, becomes apparent when considering the close interplay between inflammation and tissue regeneration within the dental pulp. Identification and characterization of the molecular and cellular events associated with pulpal inflammation is crucial to the development of improved approaches to vital pulp therapy and regeneration of the tissues.
Initiation of inflammatory and immune responses in the pulp reflects the injurious challenge to the tissue, a major determinant of which will be bacterial infection, although chemicals and materials used in restorative dentistry may also contribute to these responses. The importance of bacteria in driving pulpal inflammation is reflected in its exacerbation when the bacterial challenge persists. It has long been a significant challenge to correlate the clinical and histologic features of pulpal inflammation, which is of critical importance in determining when the step from reversible to irreversible pulpitis occurs with its consequent impact on clinical management of the tooth. The low-compliance environment of the dentin-pulp complex, which constrains tissue swelling, likely contributes to the control of the extent and intensity of the inflammatory response. Uncontrolled inflammatory responses associated with irreversible pulpitis severely compromise the opportunities for tissue regeneration within the dentin-pulp complex and drive treatment-planning decisions. There would thus be considerable merit in developing therapeutic strategies to control inflammatory/immune processes in the pulp, while recognizing that an inflammatory response of short-lived duration and intensity is a fundamental defense response for any tissue in the body. Microbial control in the carious tooth clearly provides a prime mode of dampening the inflammatory responses, but targeting signaling cascades, including the Toll-like receptors, nuclear factor κβ activation, and transcription pathways, such as those involving p38 MAP-kinase, may also have merit. Proresolving lipid mediators, including lipoxins, resolvins, and protectins, are important in the suppression of inflammation and may also provide valuable targets. The broad range of anti-inflammatory peptides and other molecules being developed within the pharmaceutical industry may have potential application in the diseased tooth and thereby provide more generic solutions to sourcing such drugs. Focus on the control of inflammation, and the associated challenge from carious bacteria, offers immense potential to significantly improve opportunities for successful vital pulp therapy and facilitate pulpal regeneration.
Cellular events in dentin-pulp regeneration
Development of effective strategies for pulpal regeneration requires both consideration and understanding of the behavior of the cells of the pulp in health as well as disease. Many aspects of regeneration within the dentin-pulp complex seem to recapitulate developmental events, albeit with a lesser degree of regulatory control. This aspect perhaps highlights the great adaptability of the dentin-pulp complex to respond to environmental changes and its strong regenerative potential. Fundamental to this responsiveness is the odontoblast, which can mediate defense as well as secretory roles. These cells represent a unique population of cells that survive for the life of a tooth in the absence of injury. Following terminal differentiation during tooth development, these cells become postmitotic in nature, and have an active synthetic and secretory role during primary dentinogenesis as the crown and root of the tooth are laid down. Subsequently, during secondary dentinogenesis their activity is significantly downregulated and they become largely quiescent, slowly secreting secondary dentin. However, odontoblasts may become upregulated in response to injury, secreting a tertiary dentin matrix to repair and regenerate the tissue and thereby restoring the tooth’s structural integrity. This adaptability of the odontoblast makes it an excellent candidate target for regenerative strategies in the treatment of dental disease.
Tertiary dentinogenesis in fact represents a range of cellular responses, which are dependent on the severity of the injury to the tooth ( Fig. 3 ). For injury of lesser intensity, such as during early caries, tooth wear, and so forth, a response of reactionary dentinogenesis may be seen. In such situations, the primary odontoblasts responsible for dentin formation during tooth development will survive, their activity being upregulated to secrete a reactionary tertiary dentin matrix. This matrix increases the distance between the cells and the injurious challenge, and protects the pulp from greater injury. As the injury intensifies, however, the survival of the odontoblasts beneath the site of injury is increasingly compromised, and eventually cellular death can occur. If the tissue environment is conducive in terms of control of the microbial and inflammatory challenges, a new generation of odontoblast-like cells may differentiate from stem/progenitor cells from within the pulp and secrete a reparative tertiary dentin matrix. A specific example of such reparative dentinogenesis is the dentin-bridge formation seen at sites of pulpal exposure after direct pulp-capping interventions. The complexity of cellular events differs significantly between reactionary and reparative dentinogenesis. Reactionary dentinogenesis simply represents the upregulation of existing primary odontoblasts from their quiescent state following primary dentinogenesis, whereas reparative dentinogenesis is more complex and requires the recruitment and differentiation of stem/progenitor cells before upregulation of the newly differentiated odontoblast-like cells for reparative dentine secretion.
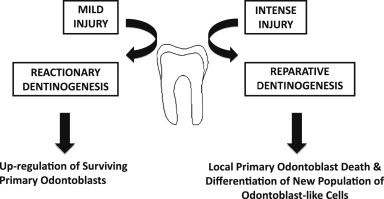
Reparative dentinogenesis clearly is important in both providing a barrier to further injurious cellular challenge and helping to restore the tooth’s structural integrity. As the matrix is secreted by a new generation of odontoblast-like cells, there will commonly not be tubular continuity with the dentinal tubules of the primary dentin, thereby increasing the role of this matrix as a protective barrier. In fact, the morphology of reparative dentin can be very variable in terms of its degree of tubularity, which has consequences for the permeability of the tissue and perhaps also reflects the pathologic nature of the secretion. The tight control of events leading to odontoblast differentiation during tooth development will not necessarily be replicated during reparative dentinogenesis and, importantly, the cells differentiating to odontoblast-like cells may not all be of the same embryonic origin or developmental state as those giving rise to primary odontoblasts. Over the last decade there has been considerable interest in the presence of stem cells in pulp and their potential application to regenerative medicine, in both the tooth and other tissues of the body. Several populations of stem cells have been reported in the pulp, including dental pulp stem cells, stem cells from the apical part of the papilla, and stem cells from human exfoliated deciduous teeth, and there is still much to learn about these various populations in terms of their homogeneity, derivation, and responses. An important question to address is whether these represent mesenchymal stem cells recruited through the circulation from sites outside the tooth and if their exposure to the niche environment within the pulp provides their phenotypic characteristics. Recent evidence supports such an origin for some cells involved in odontoblast-like cell differentiation, which has significant implications for tissue-engineering strategies in the dentin-pulp complex. It is possible that the neural-crest origin of odontoblasts may not be critical to the differentiation of odontoblast-like cells and the reparative dentin matrix they secrete. Pulp tissue engineering clearly has immense potential to have an impact on the future direction of endodontics, but unlike regenerative approaches exploiting the presence of cells in residual pulp tissue, tissue engineering requires identification of suitable stem-cell sources for the engineering of tissue constructs. SHED cells provide a source of autologous cells that may be harvested noninvasively, and their seeding in a polylactic acid polymeric scaffold within a tooth slice has allowed engineering of pulp tissue resembling that seen physiologically. Isolated dental pulp cells seeded with angiogenic growth factors in a collagen scaffold within a pulpless tooth chamber have been reported to allow tissue generation, with evidence of revascularization after in vivo implantation. Such tissue-engineering approaches offer exciting potential for the future of endodontics, but regenerative strategies based on the concept of pulpotomy provide significant potential for many patients if the management of cases can be better targeted in terms of controlling the pulpal environment to be more conducive to regeneration.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


