The search for more accessible mesenchymal stem cells than those found in bone marrow has propelled interest in dental tissues. Human dental stem/progenitor cells (collectively termed dental stem cells [DSCs]) that have been isolated and characterized include dental pulp stem cells, stem cells from exfoliated deciduous teeth, stem cells from apical papilla, periodontal ligament stem cells, and dental follicle progenitor cells. Common characteristics of these cell populations are the capacity for self-renewal and the ability to differentiate into multiple lineages. In vitro and animal studies have shown that DSCs can differentiate into osseous, odontogenic, adipose, endothelial, and neural-like tissues.
- •
The search for more accessible mesenchymal stem cells than those found in bone marrow has propelled interest in dental tissues, which are rich sources of stem cells. Human dental stem/progenitor cells (collectively termed dental stem cells [DSCs]) that have been isolated and characterized include dental pulp stem cells, stem cells from exfoliated deciduous teeth, stem cells from apical papilla, periodontal ligament stem cells, and dental follicle progenitor cells.
- •
The common characteristics of these cell populations are the capacity for self-renewal and the ability to differentiate into multiple lineages (multipotency). In vitro and animal studies have shown that DSCs can differentiate into osseous, odontogenic, adipose, endothelial, and neural-like tissues.
- •
In recent studies, third molar dental pulp somatic cells have been reprogrammed to become induced pluripotent stem cells, and dental pulp pluripotentlike stem cells have been isolated from the pulps of third molar teeth.
Introduction
The aim of regenerative medicine and tissue engineering is to replace or regenerate human cells, tissue or organs, to restore or establish normal function. The 3 key elements for tissue engineering are stem cells, scaffolds, and growth factors. Cell-based therapies are integral components of regenerative medicine that exploit the inherent ability of stem cells to differentiate into specific cell types. The extension of basic stem cell science into translational therapies is already well established with artificial skin therapies, whereas research is ongoing for cell-based therapies to target other diseases, including diabetes, atherosclerosis, and neurodegenerative diseases. The search for more accessible mesenchymal stem cells (MSCs) than those found in bone marrow has propelled interest in dental tissues, which are rich sources of stem cells. This article provides an overview of stems cells and then focuses on dental stem cells (DSCs) and how recent developments have the potential to greatly impact the way DSCs might be used in future regenerative medicine applications that include regenerative endodontic therapies.
Stem cells
General Characteristics
Stem cells are undifferentiated embryonic or adult cells that continuously divide. A fundamental property of stem cells is self-renewal or the ability to go through numerous cycles of cell division while maintaining the undifferentiated state ( Box 1 ). In addition, stem cells produce intermediate cell types (called progenitor or precursor cells) that have the capacity to differentiate into different cell types and generate complex tissues and organs. Differentiation occurs when a stem cell acquires the features of a specialized cell (eg, odontoblast).
| Undifferentiated cells | Have not developed into a specialized cell type |
| Long-term self-renewal | The ability to go through numerous cycles of cell division while maintaining the undifferentiated state |
| Production of progenitor cells | Capacity to differentiate into specialized cell types (eg, odontoblast, osteoblast, adipocyte, fibroblast) |
Stem cells can be either embryonic or adult (postnatal). Thomson and colleagues first reported human embryonic stem cell lines in 1998. Embryonic stem cells are isolated from the blastocyst during embryonic development and give rise to the 3 primary germ layers: ectoderm, endoderm, and mesoderm. These cells are totipotent or pluripotent with an unlimited capacity to differentiate and can develop into each of the more than 200 cell types of the adult body ( Box 2 ).
| Embryonic stem cells from inner cell mass of 3- to 5-day embryo (blastocyst) | Totipotent | Can give rise to all the cell types of the body, including those cells making the extraembryonic tissues (eg, placenta) |
| Unlimited capacity to divide | ||
| Embryonic stem cells Induced pluripotent stem cells |
Pluripotent | Can form derivatives of all the embryonic germ layers (ectoderm, mesoderm, and endoderm) from a single cell |
| Can give rise to all of the various cell types of the body | ||
| Adult stem cells (postnatal) | Multipotent | Can give rise to more than one cell type of the body |
| Induced pluripotent stem cells | Pluripotent | Derived from somatic cells |
Adult stem cells exist throughout the body in different tissues, including bone marrow, brain, blood vessels, liver, skin, retina, pancreas, peripheral blood, muscle, adipose tissue, and dental tissues. They are localized to specific niches where the regulation of stem cell proliferation, survival, migration, fate, and aging occur. Whether cells undergo either prolonged self-renewal or differentiation depends on intrinsic signals modulated by extrinsic factors in the stem cell niche. An adult stem cell can divide and create another cell like itself, and also a cell more differentiated than itself, but the capacity for differentiation into other cell types is limited. This capability is described as being multipotent and is a distinguishing feature of adult stem cells compared with the pluripotency of embryonic stem cells. Although early research suggested that adult stem cells were limited in the types of tissues they produced, it is increasingly apparent that adult stem cells have greater plasticity than previously thought and can generate a tissue different to the site from which they were originally isolated. An example with potential clinical applications is the ability of dental pulp cells to generate heart tissue in rats.
MSCs
In 1963, hematopoietic stem cells giving rise to blood cells were identified in bone marrow. Since then, it has been established that bone marrow is also the primary source for multipotent MSCs. Bone marrow MSCs (BMMSCs) can differentiate into osteogenic, chondrogenic, adipogenic, myogenic, and neurogenic lineages. MSCs are found in many other tissues in the body, including umbilical cord blood, adipose tissue, adult muscle, and dental tissues ; are capable of differentiating into at least 3 cell lineages: osteogenic, chondrogenic, and adipogenic ; and can also differentiate into other lineages, such as odontogenic, when grown in a defined microenvironment in vitro.
Definitive information on the location and distribution of MSCs is still being elucidated. However, it has been shown that MSCs can be found around blood vessel walls and perineurium as demonstrated by the immuno-colocalization of STRO-1/CD146 stem cell markers. These observations have led to the proposal that MSCs arise from a perivascular stem cell niche that provides an environment allowing the cells to retain their stemness. Crisan and colleagues demonstrated that human perivascular cells from diverse and multiple human tissues give rise to multi-lineage progenitor cells that exhibit the features of MSCs. Perivascular progenitor/stem cells can also proliferate in response to odontoblast injury by cavity preparation under ex vivo tooth culture conditions.
Isolation, Identification, and Differentiation of MSCs
A fundamental approach to isolate MSCs in tissue samples involves the enzymatic digestion of tissue followed by the growth of isolated cells (expansion) in medium rich in growth factors. The isolation of more immature stem cells involves a multistep explant approach whereby pieces of tissue are first cultured until progenitor cells grow after which enzymatic digestion and expansion in media proceed.
The identification of MSCs uses a series of in vitro tests. Colony-forming assays are used to confirm clonogenicity (the ability to generate identical stem cells with the appropriate cell morphology), which is a consistent characteristic of MSCs. Phenotypic assays evaluate cell morphology or shape (eg, fibroblastic when flat and elongated) and cell behavior (eg, secretory). The possession of one or several cell surface markers found on cells in representative tissues is evaluated by flow cytometry, which sorts cells with specific surface protein, such as STRO-1, found on stem cells that can differentiate into multiple mesenchymal lineages, including dental pulp cells ( Fig. 1 C, F, I). DSCs can also express specific proteins associated with endothelium (CD106, CD146), perivascular tissues (α-smooth muscle actin, CD146, 3G5), bone, dentin and cementum (bone morphogenic protein [BMP], alkaline phosphatase, osteonectin, osteopontin, and bone sialoprotein), and fibroblasts (type I and III collagen).
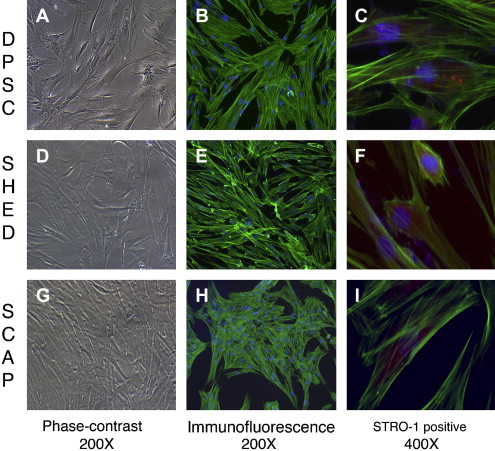
In vitro functional assays test putative MSCs for multipotency by confirming that differentiated cells demonstrate the appropriate phenotypic characteristics. Accordingly, the in vitro confirmation of the multipotency of dental pulp stem cells (DPSCs) can be demonstrated by the evidence of odontoblastlike differentiation (verified by the deposition of mineralized matrix and positive staining for dentin sialophosphoprotein), adipogenic differentiation (by the accumulation of lipid vacuoles), chondrogenic differentiation (by the production of collagen type II), and neurogenic differentiation (by neuronal-cell morphologies and markers).
In vivo functional assays are used to confirm that stem cells implanted into a new environment (eg, immunodeficient mice) successfully integrate with adjacent cells, survive, and function as differentiated cells. Several studies have demonstrated the formation of new pulp and dentinlike tissues following the insertion of DSCs seeded onto scaffolds in emptied human root canals or dentin disks embedded into immunocompromised mice; the resulting dentinogenesis is accomplished by odontoblastlike cells derived from MSCs.
Storage of Stem Cells
Adult stem cells can be obtained from individuals at any stage in life and, therefore, can provide a source of cells for autologous transplants. Such procedures invariably require stem cell storage, which is achieved by cryopreservation in liquid nitrogen (−196°C). Stem cells can survive these low temperatures as long as they are dispersed in cryprotectants. Human periodontal ligament stem cells (PDLSCs) have been successfully recovered after cryopreservation for 6 months; although the number of colonies was less than for fresh PDLSCs, the proliferation rate was similar. Similarly, stem cells isolated from human third molar teeth and cryopreserved for at least 1 month retained STRO-1 marker expression and the potential to proliferate into neurogenic, adipogenic, osteogenic/odontogenic, myogenic, and chondrogenic pathways in inductive media. Cryopreservation of intact teeth provides another potential storage method that can allow later extraction of stem cells demonstrating similar behavior as stem cells extracted from fresh teeth.
Stem cells
General Characteristics
Stem cells are undifferentiated embryonic or adult cells that continuously divide. A fundamental property of stem cells is self-renewal or the ability to go through numerous cycles of cell division while maintaining the undifferentiated state ( Box 1 ). In addition, stem cells produce intermediate cell types (called progenitor or precursor cells) that have the capacity to differentiate into different cell types and generate complex tissues and organs. Differentiation occurs when a stem cell acquires the features of a specialized cell (eg, odontoblast).
| Undifferentiated cells | Have not developed into a specialized cell type |
| Long-term self-renewal | The ability to go through numerous cycles of cell division while maintaining the undifferentiated state |
| Production of progenitor cells | Capacity to differentiate into specialized cell types (eg, odontoblast, osteoblast, adipocyte, fibroblast) |
Stem cells can be either embryonic or adult (postnatal). Thomson and colleagues first reported human embryonic stem cell lines in 1998. Embryonic stem cells are isolated from the blastocyst during embryonic development and give rise to the 3 primary germ layers: ectoderm, endoderm, and mesoderm. These cells are totipotent or pluripotent with an unlimited capacity to differentiate and can develop into each of the more than 200 cell types of the adult body ( Box 2 ).
| Embryonic stem cells from inner cell mass of 3- to 5-day embryo (blastocyst) | Totipotent | Can give rise to all the cell types of the body, including those cells making the extraembryonic tissues (eg, placenta) |
| Unlimited capacity to divide | ||
| Embryonic stem cells Induced pluripotent stem cells |
Pluripotent | Can form derivatives of all the embryonic germ layers (ectoderm, mesoderm, and endoderm) from a single cell |
| Can give rise to all of the various cell types of the body | ||
| Adult stem cells (postnatal) | Multipotent | Can give rise to more than one cell type of the body |
| Induced pluripotent stem cells | Pluripotent | Derived from somatic cells |
Adult stem cells exist throughout the body in different tissues, including bone marrow, brain, blood vessels, liver, skin, retina, pancreas, peripheral blood, muscle, adipose tissue, and dental tissues. They are localized to specific niches where the regulation of stem cell proliferation, survival, migration, fate, and aging occur. Whether cells undergo either prolonged self-renewal or differentiation depends on intrinsic signals modulated by extrinsic factors in the stem cell niche. An adult stem cell can divide and create another cell like itself, and also a cell more differentiated than itself, but the capacity for differentiation into other cell types is limited. This capability is described as being multipotent and is a distinguishing feature of adult stem cells compared with the pluripotency of embryonic stem cells. Although early research suggested that adult stem cells were limited in the types of tissues they produced, it is increasingly apparent that adult stem cells have greater plasticity than previously thought and can generate a tissue different to the site from which they were originally isolated. An example with potential clinical applications is the ability of dental pulp cells to generate heart tissue in rats.
MSCs
In 1963, hematopoietic stem cells giving rise to blood cells were identified in bone marrow. Since then, it has been established that bone marrow is also the primary source for multipotent MSCs. Bone marrow MSCs (BMMSCs) can differentiate into osteogenic, chondrogenic, adipogenic, myogenic, and neurogenic lineages. MSCs are found in many other tissues in the body, including umbilical cord blood, adipose tissue, adult muscle, and dental tissues ; are capable of differentiating into at least 3 cell lineages: osteogenic, chondrogenic, and adipogenic ; and can also differentiate into other lineages, such as odontogenic, when grown in a defined microenvironment in vitro.
Definitive information on the location and distribution of MSCs is still being elucidated. However, it has been shown that MSCs can be found around blood vessel walls and perineurium as demonstrated by the immuno-colocalization of STRO-1/CD146 stem cell markers. These observations have led to the proposal that MSCs arise from a perivascular stem cell niche that provides an environment allowing the cells to retain their stemness. Crisan and colleagues demonstrated that human perivascular cells from diverse and multiple human tissues give rise to multi-lineage progenitor cells that exhibit the features of MSCs. Perivascular progenitor/stem cells can also proliferate in response to odontoblast injury by cavity preparation under ex vivo tooth culture conditions.
Isolation, Identification, and Differentiation of MSCs
A fundamental approach to isolate MSCs in tissue samples involves the enzymatic digestion of tissue followed by the growth of isolated cells (expansion) in medium rich in growth factors. The isolation of more immature stem cells involves a multistep explant approach whereby pieces of tissue are first cultured until progenitor cells grow after which enzymatic digestion and expansion in media proceed.
The identification of MSCs uses a series of in vitro tests. Colony-forming assays are used to confirm clonogenicity (the ability to generate identical stem cells with the appropriate cell morphology), which is a consistent characteristic of MSCs. Phenotypic assays evaluate cell morphology or shape (eg, fibroblastic when flat and elongated) and cell behavior (eg, secretory). The possession of one or several cell surface markers found on cells in representative tissues is evaluated by flow cytometry, which sorts cells with specific surface protein, such as STRO-1, found on stem cells that can differentiate into multiple mesenchymal lineages, including dental pulp cells ( Fig. 1 C, F, I). DSCs can also express specific proteins associated with endothelium (CD106, CD146), perivascular tissues (α-smooth muscle actin, CD146, 3G5), bone, dentin and cementum (bone morphogenic protein [BMP], alkaline phosphatase, osteonectin, osteopontin, and bone sialoprotein), and fibroblasts (type I and III collagen).
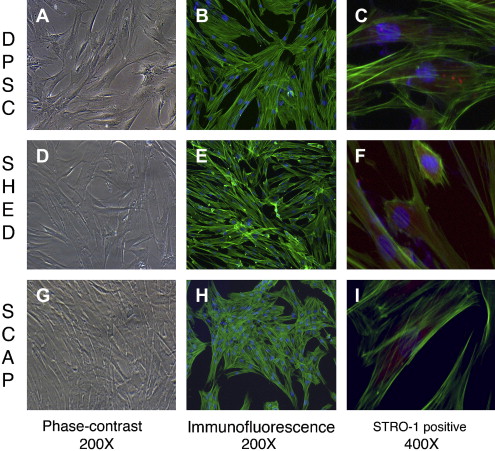
In vitro functional assays test putative MSCs for multipotency by confirming that differentiated cells demonstrate the appropriate phenotypic characteristics. Accordingly, the in vitro confirmation of the multipotency of dental pulp stem cells (DPSCs) can be demonstrated by the evidence of odontoblastlike differentiation (verified by the deposition of mineralized matrix and positive staining for dentin sialophosphoprotein), adipogenic differentiation (by the accumulation of lipid vacuoles), chondrogenic differentiation (by the production of collagen type II), and neurogenic differentiation (by neuronal-cell morphologies and markers).
In vivo functional assays are used to confirm that stem cells implanted into a new environment (eg, immunodeficient mice) successfully integrate with adjacent cells, survive, and function as differentiated cells. Several studies have demonstrated the formation of new pulp and dentinlike tissues following the insertion of DSCs seeded onto scaffolds in emptied human root canals or dentin disks embedded into immunocompromised mice; the resulting dentinogenesis is accomplished by odontoblastlike cells derived from MSCs.
Storage of Stem Cells
Adult stem cells can be obtained from individuals at any stage in life and, therefore, can provide a source of cells for autologous transplants. Such procedures invariably require stem cell storage, which is achieved by cryopreservation in liquid nitrogen (−196°C). Stem cells can survive these low temperatures as long as they are dispersed in cryprotectants. Human periodontal ligament stem cells (PDLSCs) have been successfully recovered after cryopreservation for 6 months; although the number of colonies was less than for fresh PDLSCs, the proliferation rate was similar. Similarly, stem cells isolated from human third molar teeth and cryopreserved for at least 1 month retained STRO-1 marker expression and the potential to proliferate into neurogenic, adipogenic, osteogenic/odontogenic, myogenic, and chondrogenic pathways in inductive media. Cryopreservation of intact teeth provides another potential storage method that can allow later extraction of stem cells demonstrating similar behavior as stem cells extracted from fresh teeth.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


