Periodontal and endodontic diseases are inflammatory responses leading to periodontal and pulpal tissue loss. Regenerative therapies aim to restore the lost structures to vitality and function. Various materials and treatments methods have been used such as bone grafts, guided tissue regeneration, enamel matrix derivatives, growth and differentiation factors, and stem cells. Although the current materials and methods demonstrated adequate clinical results, true and complete biological tissue regeneration is not yet attainable. The current article reviews chronologically the materials and methods used in periodontal and endodontic regeneration highlighting their clinical success and shortcomings, and discussing future directions in regenerative therapy.
- •
Periodontal and endodontic diseases are inflammatory responses to microorganisms leading to periodontal and pulpal tissue damage and loss. Regenerative therapies aim to restore the lost structures to vitality and function.
- •
Various materials and treatments methods, such as bone grafts, guided tissue regeneration, enamel materials derivatives, growth and differentiation factors, and stem cells research, have been used.
- •
Although the current materials and methods demonstrated favorable clinical results, true and complete biological regeneration of the damaged or injured structures is not yet attainable.
Introduction
Periodontal disease is a destructive inflammatory process affecting the periodontal attachment apparatus of the teeth, leading to irreversible loss of bone and periodontal ligament (PDL). Similarly in endodontics, a progressive injury (ie, caries) to the dental pulp stimulates an inflammatory response leading to pulpal necrosis and periapical tissue involvement manifested in periradicular tissue breakdown. Various treatment methods have been proposed for the treatment of periodontal disease. In the 1950s and 1960s, treatments ranged from conservative therapy, such as curettage and open flap debridement, to surgical treatment procedures, such as gingivectomy, root amputation, hemi-section, and tunneling. Regardless of the treatment method used, healing occurred by repair (ie, long junctional epithelium) rather than regeneration of the lost tissues of the periodontal attachment apparatus. The introduction of bone grafts in the 1970s and 1980s and the concept of guided tissue regeneration (GTR) offered new treatment options to regenerate lost periodontal tissues. In the 1990s and 2000s, enamel matrix derivatives (EMDs), platelet-derived growth factor (PDGF), insulinlike growth factor (IGF-I), and bone morphogenetic proteins (BMPs) have given a new dimension to periodontal regeneration with promising clinical results. The aim of periodontal treatment is complete regeneration of the lost or damaged tissues. Despite the success of the above-mentioned materials and methods, this aim has been partially but not completely established. At present, stem cells (SCs) represent a new era in regenerative therapy with the aim of complete regeneration. This article chronologically reviews the regenerative therapies used in periodontal and endodontic fields with emphasis on periodontal regeneration.
Healing
Endodontic Repair Versus Regeneration
In pulpal injury, if the original odontoblasts survive and react by secreting tertiary dentin matrices, complete regeneration and return of the damaged vital pulp tissue to its normal form and function is possible via reactionary dentinogenesis. Conversely, reparative dentin formation occurs in response to deep dentinal caries and represents a much more complex sequence of biologic events beginning with apoptosis of the odontoblasts in the area of injury, including progenitor cell recruitment and differentiation. When the pulp is exposed in advanced lesions, reparative dentinogenesis may result in dentin bridge formation, which restores only the functional integrity of the pulpodentin complex. In the case of a periradicular lesion due to pulpal necrosis, usually the lesion would heal to form and function after conventional endodontic therapy. However, in some instances, the lesion may persist or not completely heal despite adequate therapy. Generally, a persistent periapical radiolucent lesion would indicate ongoing disease. On occasion, unresolved periapical radiolucency may represent healing by remnants of the lesion, which may be mistaken as a sign of failed endodontic treatment. These types of lesions usually occur when the lesion penetrates both the buccal and lingual cortical plates (ie, communicating lesion). In such cases, the form and functional healing of the periradicular tissues are replaced by a fibrous tissue repair of the area, which persists as a radiolucency alluding to persistent disease of the apical region.
Periodontal Repair Versus Regeneration
Periodontally, repair is healing of a wound by tissue that does not fully restore the architecture or the function of the lost part as in healing by long junctional epithelium. Regeneration constitutes the reproduction of a lost or injured part. Periodontally, GTR is a procedure that attempts to regenerate the lost periodontal structures through differential tissue responses by using a barrier (ie, membrane). Thus, restoration of the periodontal apparatus occurs by the regeneration of connective tissue, cementum, and alveolar bone rather than epithelium. Healing by regeneration is favorable compared with repair because the goal of periodontal therapy is to restore the periodontal tissues to their original biological structure. Many materials and methods have been used to accomplish regeneration, including bone grafts, GTR, EMDs, growth factors, and SCs.
Healing
Endodontic Repair Versus Regeneration
In pulpal injury, if the original odontoblasts survive and react by secreting tertiary dentin matrices, complete regeneration and return of the damaged vital pulp tissue to its normal form and function is possible via reactionary dentinogenesis. Conversely, reparative dentin formation occurs in response to deep dentinal caries and represents a much more complex sequence of biologic events beginning with apoptosis of the odontoblasts in the area of injury, including progenitor cell recruitment and differentiation. When the pulp is exposed in advanced lesions, reparative dentinogenesis may result in dentin bridge formation, which restores only the functional integrity of the pulpodentin complex. In the case of a periradicular lesion due to pulpal necrosis, usually the lesion would heal to form and function after conventional endodontic therapy. However, in some instances, the lesion may persist or not completely heal despite adequate therapy. Generally, a persistent periapical radiolucent lesion would indicate ongoing disease. On occasion, unresolved periapical radiolucency may represent healing by remnants of the lesion, which may be mistaken as a sign of failed endodontic treatment. These types of lesions usually occur when the lesion penetrates both the buccal and lingual cortical plates (ie, communicating lesion). In such cases, the form and functional healing of the periradicular tissues are replaced by a fibrous tissue repair of the area, which persists as a radiolucency alluding to persistent disease of the apical region.
Periodontal Repair Versus Regeneration
Periodontally, repair is healing of a wound by tissue that does not fully restore the architecture or the function of the lost part as in healing by long junctional epithelium. Regeneration constitutes the reproduction of a lost or injured part. Periodontally, GTR is a procedure that attempts to regenerate the lost periodontal structures through differential tissue responses by using a barrier (ie, membrane). Thus, restoration of the periodontal apparatus occurs by the regeneration of connective tissue, cementum, and alveolar bone rather than epithelium. Healing by regeneration is favorable compared with repair because the goal of periodontal therapy is to restore the periodontal tissues to their original biological structure. Many materials and methods have been used to accomplish regeneration, including bone grafts, GTR, EMDs, growth factors, and SCs.
Regenerative therapy
Bone Grafts
Bone grafts are used as fillers in periodontal defects and aid in healing by repair. Bone grafts are used because of their osteogenic (autograft), osteoinductive (allograft), or osteoconductive (xenograft/alloplast) properties. Osteogenic materials stimulate the formation of new bones because they contain bone-forming cells and progenitor cells. Osteoinductive materials stimulate progenitor SCs in the surrounding tissue immediately adjacent to the defect to regenerate the lost structures. In contrast, osteoconductive materials serve as scaffolds for bone growth within the existing bony walls and passively promote healing. Although bone grafts demonstrated adequate clinical results, the healing mainly occurred by repair (long junctional epithelium), rather than regeneration of the PDL ( Fig. 1 ).
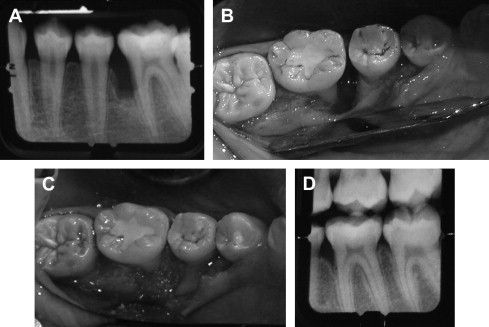
Bone grafts are also used in the treatment of apical periodontitis and more specifically, in periradicular surgery. Freeze-dried bone allografts (FDBA) were used in osseous defects after the removal of periapical lesions. The results showed that FDBA had an osteogenic potential and, for the most part, bone regeneration occurred. Although the previously mentioned studies were beneficial in healing of periodontal and periradicular defects, complete true regeneration did not occur.
Guided Tissue Regeneration
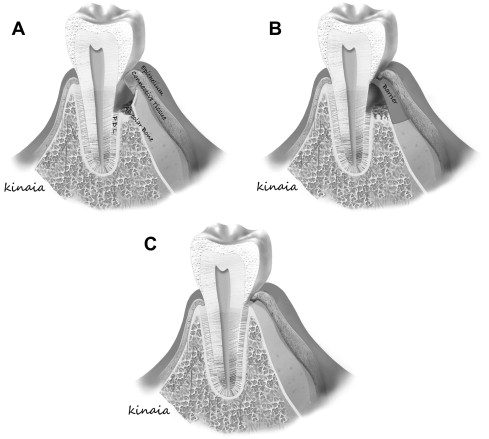
Melcher suggested that there are 4 different cell types dictating the type of periodontal healing that occurs. These cells originate from the gingival epithelial tissue, lamina propria of connective tissue, alveolar bone, and PDL. Cells derived from PDL and bone posses the potential to heal by true regeneration when compared with cells from lamina propria of gingiva or gingival epithelial tissue that heal by repair ( Fig. 2 ). Understanding barrier mediated selective cell repopulation gave rise to the concept of epithelial exclusion to restore lost periodontal tissue and obtain new attachment. Bowers and colleagues published a histologic report on the formation of new periodontal attachment apparatus in humans. The study included teeth with intrabony defects that needed to be extracted because of advanced periodontal disease. The investigators performed flap curettage, crown removal, and submersion of the vital root beneath the mucosa with biopsies obtained at 6 months after treatment. The submersion of the teeth allowed epithelial exclusion similar to GTR. New attachment occurred in the submerged group indicating regeneration. Healing by long junctional epithelium was observed on the nonsubmerged teeth, indicating repair. Further, regeneration of new attachment apparatus, cementum, and bone was more likely in submerged, intrabony grafted defects with demineralized FDBA. New attachment in grafted sites measured 1.76 mm compared with 0.76 mm in nongrafted sites. The investigators concluded that healing with a new attachment was more predictable for submerged teeth and more likely when a bone graft was added. The literature demonstrated that GTR was biologically possible with promising clinical results in intrabony and furcation defects.
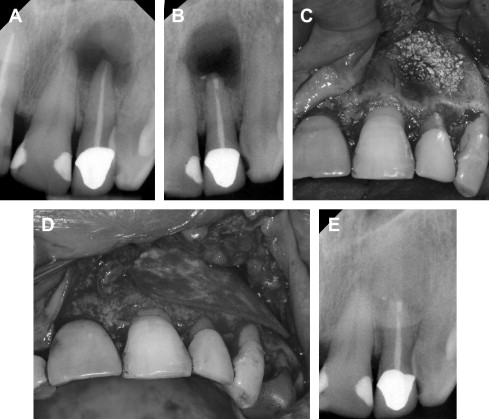
Although the concept of GTR was primarily established in periodontal regeneration, it also has been applied in surgical endodontic treatment ( Fig. 3 ). A recent systematic review by Tsesis and colleagues evaluated the efficacy of GTR in endodontic surgery. They found that large communicating lesions healed better with GTR compared with those without GTR ( P = .02). However, GTR in small confined lesions was of no added benefit ( P = .27). The use of a resorbable membrane was more favorable than nonresorbable membranes ( P = .02), a finding that was similar to that obtained in the regeneration of periodontal furcation defects. Therefore, when a periapical lesion is confined, GTR may not be necessary. If there is a large communication of the periapical lesion, GTR would be of potential benefit. Despite the success of GTR in periodontal and endodontic treatments, complete regeneration of the periodontal attachment apparatus is not always predictable. Thus, advances in molecular biology, such as EMDs and growth factors, offer a new area of research with the hope of complete regeneration.
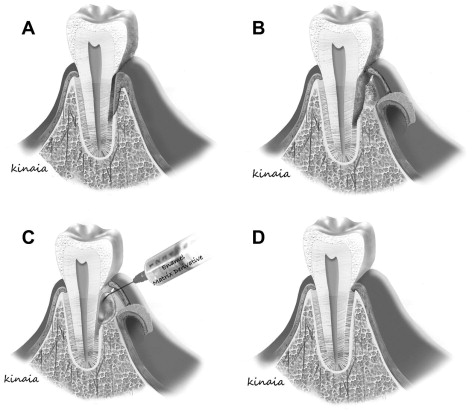
Enamel Matrix Derivatives
Advances in molecular biology set the stage for a new era in periodontal regeneration for complete regeneration ( Fig. 4 ). Studies reported that treatment of intrabony defects with EMDs led to decreased probing depth, increased clinical attachment level (CAL), increased bone-fill, and periodontal regeneration. EMD contains amelogenins among other enamel matrix proteins that mimic the cementogenesis process during root formation. These proteins, in primate studies, have demonstrated stimulation of the surrounding undifferentiated mesenchymal cells into cementoblasts to form acellular cementum, a process similar to the formation of the inner layer of Hertwig epithelial root sheath during tooth development. Once the cementum is formed, collagen fibers attach from the adjacent PDL leading to the restoration of the periodontal attachment apparatus. Although not consistent, EMD creates a favorable environment at the cellular level for periodontal regeneration by improving the attachment as well as differentiation of PDL fibroblasts compared with gingival fibroblasts. Similarly, the amelogenins are involved in the differentiation of odontoblasts during development indicating that they may play a role in odontogenesis. Nakamura and colleagues examined the effect of EMD on pulpal wound healing in an animal study using premolars. They found that EMD formed dentinlike hard tissue with the presence of formative cells outlining the pulpal wound. EMD had a 2-fold better reparative potential of the pulpal wound compared with the control (calcium-hydroxide) group. At the microbiological level, EMD selectively inhibited the growth of gram-negative pathogens although exhibiting no effect on gram-positive pathogens. EMD mainly creates a positive environment, but it does not contain a specific growth factor that can be useful to enhance regeneration. More recent advances in regenerative therapies include the use of growth and differentiation factors for periodontal and endodontic regeneration.
Growth and Differentiation Factors
Growth and differentiation factors play an important role in regulating wound-healing events, such as chemotaxis, cell adhesion, proliferation, and differentiation. These factors include platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factors (TGF) α and β, acidic and basic fibroblast growth factors, epidermal growth factor, IGF-I, IGF-II, cementum-derived growth factor, parathyroid hormone-related protein, and BMPs. See the article by Kim and colleagues in this issue for further details on growth and differentiation factors.
At present, the most used factors are PDGF, IGF, and BMPs. PDGFs are dimeric glycoproteins comprising 2 A (-AA), 2 B (-BB) chains, or a combination of the 2 (-AB) chains. PDGF-BB has been used in intrabony periodontal defects with significant improvement in clinical outcomes demonstrated as CAL gain, bone growth, and percentage bone fill. IGF is a protein with 2 ligands (IGF-1 and IGF-2). Howell and colleagues reported CAL gain, periodontal probing depth (PPD) reduction, and bone gain with use of a combination of IGF-1 and PDGF-BB. Further, a pulp-capping study in rat molars by Lovschall and colleagues reported a positive effect of IGF-1 in dentin repair. IGF-1 improved the reparative dentinogenesis in injured dental pulps. Contrary to the Lovschall study, Regan and colleagues evaluated the use of the PDGF and IGF combination in periapical surgery and reported no regeneration of the periapical tissues. PDGF and IGF have shown promising results in periodontal regeneration but their results are controversial in endodontic regeneration.
BMPs are differentiating factors belonging to the TGF-β superfamily. They play a major role in differentiation, cell migration, proliferation, and apoptosis. At present, there are more than 20 BMPs with BMP-2 (osteogenic protein-2 [OP-2]), BMP-3 (osteogenin), and BMP-7 (osteogenic protein-1 [OP-1]) being the most useful in regenerative therapy. In 2007, the Food and Drug Administration approved the use of INFUSE Bone Graft containing BMP in dental regeneration. BMP-2 and -3 have shown potential in correcting intrabony and furcation bone loss. However, BMP-2 has been associated with ankylosis histologically. Therefore, these molecules are generally reserved for use around implants or for guided bone regeneration. However, BMP-7 has been used successfully in periodontal regeneration with no ankylosis. Similarly, animal studies reported the differentiation of pulp cells into odontoblasts leading to the formation of osteodentin when using BMP-2 and BMP-7. The current regenerative methods have shown adequate clinical results, but complete regeneration as of yet is not predictably achievable. New research emphasizes the use of SCs with the aim and hope of complete regeneration of the original periodontal and endodontic tissues.
Stem Cells
Although growth and differentiating factors have shown positive clinical results, they possess a short biologic half-life that may limit their use in complete regeneration of lost or damaged tissues. Therefore, constant release of these factors may be essential for complete periodontal regeneration. SCs are readily present in human tissues offering new horizons for complete regeneration. Dental SCs are primarily found in the dental pulp and PDL. Three dental SC populations have been identified based on their origin: dental pulp SCs (DPSCs), SCs from human exfoliated deciduous teeth (SHED), and PDLSCs . DPSC’s and SHEDs are further discussed in the article by Sedgley and colleagues in this issue.
Several preclinical studies have been reported on PDLSCs. In 1 study, PDLSCs were isolated from extracted human third molar PDLs and transplanted into immunocompromised mice and rats. PDLSCs differentiated into cementoblastlike cells, adipocytes, and collagen-forming cells and generated cementum/PDLlike tissues. PDLSCs are similar to DPSCs and SHED in their expression of surface markers of STRO-1 and CD146/MUC18. Further, they are superior to bone mesenchymal stromal SCs (BMSSCs) in their high proliferation rate and differentiation capacity. Lin and colleagues examined human periodontium of molar teeth. The investigators used SC markers STRO-1, CD146, and CD44, and were able to identify PDLSCs in the regenerated tissue, indicating their involvement in periodontal regeneration. Another marker that has been seen in BMSSCs recruitment is stromal cell–derived factor-1 (SDF-1). A recent study by Du and colleagues, reported significant proliferation and stimulated the migration of PDLSCs at concentrations of 100 and 400 ng/mL of SDF-1. This process suggests that SDF-1 may play a role in periodontal tissue regeneration in addition to the previous cell markers mentioned. PDLSCs require a scaffold such as hydroxyapatite/tricalcium phosphate to generate periodontal tissues.
PDL progenitor cells (PDLPs) are alternative cell sources to PDLSCs. PDLPs have been shown to play a role in periodontal regeneration because both are driven from the PDL. SCs differentiate into progenitor cells, which are more developmentally committed, yet are undifferentiated in comparison to those cells that have differentiated into specialized tissue cells. Fen and colleagues examined the use of PDLPs in the treatment of human intrabony periodontal defects and compared it to PDLSCs. PDLPs were transplanted in defects measuring more than 6 mm depth in 3 patients. PDLPs were similar to PDLSCs in their high proliferation rate and multipotent differentiation, resulting in therapeutic periodontal regeneration. This study represents one of the early clinical studies examining the use of stem/progenitor cells in periodontal regeneration in humans.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


